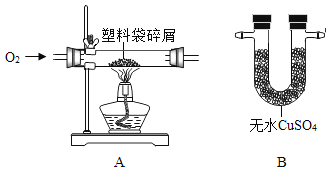
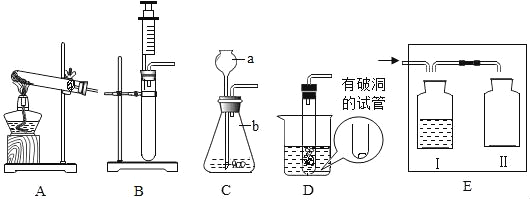
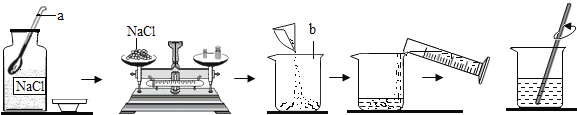
��Ŀ����
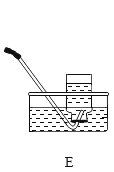
����Ŀ��С��ͬѧ����һ�����������������Ȼ�����Һ������ʵ��ʱ�������ƹ�����ͼ��ʾ��
�Իش��������⣺
��1��д��ͼ���б�����������ƣ�a_______��b_________________________________.
��2��ͼʾʵ������һ�����ԵĴ�����______��ͼʾʵ���в�������������_______��
��3��С��ͬѧҪ����80g��������Ϊ6%���Ȼ�����Һ����Ҫ��ȡˮ�����Ϊ________mL��ˮ���ܶȽ��ƿ���1g/cm3����
��4��С���ڳ����Ȼ���ʱ���Ȼ��ƺ�����Ū���ˣ���ʵ�ʳ������Ȼ��Ƶ�����Ϊ��1�����������룩��_______��
��5����һѧ���ø�6%���Ȼ�����Һ���ܶ�Ϊ��1.04��/cm3)ȥ����80����������Ϊ3%���Ȼ�����Һ������Ҫ6%��NaCl��Һ��________�ˣ�ˮ��__________ml��
��6������⣬��������Һ��������������ƫС����ԭ�������_______������ţ���
���Ȼ��ƹ��岻��
�ڳ���ʱ�����������������ͬ��ֽƬ
����ȡˮʱ�����Ӷ���
��װƿʱ����������Һ����
���𰸡�ҩ�� �ձ� ƿ��û�е����������� �����ܽ� 75.2 3.2g 40 38.46 �٢ڢ�
��������
һ�����������������Ȼ�����Һ������ʵ����Ҫ���裺���㡢��������ȡ���ܽ⡣
��1��ȡ�ù����ĩ״ҩƷ�õ�������ҩ�ף������õ��Ȼ��ƹ��嵹�뵽�ձ��У�����ȡˮ֮�����ձ��н����ܽ� ��
�ʴ�Ϊ��ҩ�ף��ձ���
��2��ȡ��ҩƷʱ��ƿ��Ҫ�����������ϣ���ֹ��ȾҩƷ��ʴ���棬����ͼ�д���֮���ǣ�ƿ��û�е����������ϣ��ܽ��Ȼ��ƵĹ����в�����Ҫ���Ͻ��裬Ŀ���ǣ������ܽ⡣
�ʴ�Ϊ��ƿ��û�е����������ϣ������ܽ⡣
��3��Ҫ����80g��������Ϊ6%���Ȼ�����Һ����Ҫ�Ȼ��Ƶ�����=80g��6%=4.8g����Ҫˮ������=80g-4.8g=75.2g��ˮ���ܶȽ��ƿ���1g/cm3����1g/ml��������Ҫˮ�����=75.2g��1g/ml=75.2ml��
�ʴ�Ϊ��75.2��
��4�������Ȼ���ʱ���Ȼ��ƺ�����Ū���ˣ���ƽ��ѭ�ĸܸ�ƽ��ԭ�����䣬����m���� gl����=��m����+m������gl���� ����ƽ�ǵȱ۸ܸˣ�����m���� =m����+m��������m����=m����- m��������Ҫ�Ȼ��Ƶ�����=80g��6%=4.8g��1�����������룬��m����=4g-0.8g=3.2g��
�ʴ�Ϊ��3.2g��
��5������Ҫ6%��NaCl��Һ������Ϊm������ϡ��ǰ�����ʵ���������ɵã�![]() ����ã�
����ã�![]() ���֣�
���֣�![]() ��
��![]() ��
��
�ʴ�Ϊ��40��38.46��
��6����������Һ�� ����������ƫС����Һ����ƫ����ܼ�ˮ�������������ƫ���ᵼ�����ʵ���������ƫС��
����������ƫС����Һ����ƫ����ܼ�ˮ�������������ƫ���ᵼ�����ʵ���������ƫС��
���Ȼ��ƹ��岻�����ᵼ��ʵ�ʳƵõ��Ȼ�������ƫС���������Ϸ����ᵼ��������������ƫС���������⣻
�ڳ���ʱ�����������������ͬ��ֽƬ���dzƵõ��Ȼ�����������ֽƬ�������ڼ���õ����������������Ȼ�������ƫС���ᵼ�����ʵ���������ƫС���������⣻
����ȡˮʱ��ˮ����������ȼ���õģ����Ӷ�����ʹʵ��Һ����ڼ���õ���ֵ ����ȡ��ˮ�ͻ�ƫ�࣬�ᵼ�����ʵ���������ƫС���������⣻
�����úõ���Һ���о�һ�ԣ�װƿʱ����������Һ��������Ӱ�����������Ĵ�С����װƿʱ����������Һ���������������⡣
�ʴ�Ϊ���٢ڢۡ�

����Ŀ������ʵ��������ܴﵽʵ��Ŀ�ĵ��ǣ� ��
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
A | ���� H2SO4 ��Һ�� NaOH ��Һ | ȡ�����μӷ�̪��Һ���۲����� |
B | ֤������������Һ��ϡ�����ܷ�Ӧ | ȡ����������Һ���ձ��У����뼸�η�̪��Һ�����ٵ���ϡ��������Һ��Ϊ��ɫ |
C | ��ȥCaCl2��Һ�е��������� | �������������� |
D | ��ȥCO�к��е�����CO2 | ͨ������������Һ�У���ͨ��Ũ������� |
A.AB.BC.CD.D