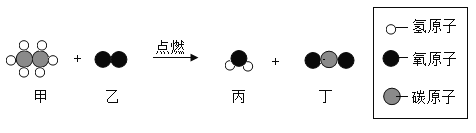
��Ŀ����
����Ŀ��С��ͬѧ����100g 10%���Ȼ�����Һ����������������ͼ��ʾ���ش��������⣺
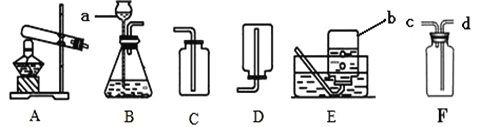
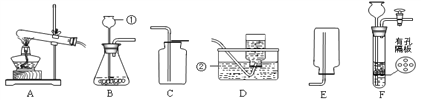
��1��ͼ����ʢ���Ȼ��ƵĹ��������������_____�����в����������________������ţ�
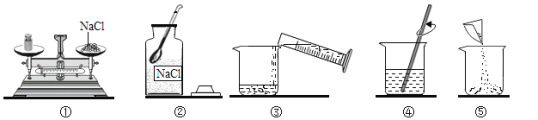
��2��������Һ����ȷ����˳��Ϊ_____________ ������ţ�
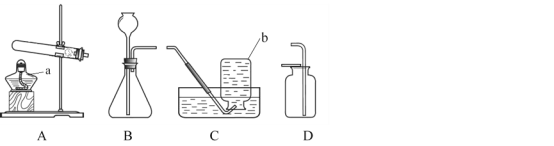
��3����ˮʱѡ�õ���Ͳ����������ʵ���_______��������ţ�
A.10ml B.25ml C.50m D.100ml
��4������⣬��ͬѧ���Ƶ���Һ������������ƫС�����ܵ�ԭ����________ ������ţ�
A. �Ȼ��ƹ��岻�� B����������������
C��װƿʱ��������Һ���� D���ܽ�ʱ�ձ��ڱ���ˮ��
��5�������С��������¼��㣺
��Ҫ����100g 10%���Ȼ��Ƶ���Һ����Ҫ��ȡʳ��______ g������ˮ_______ g��
����Ҫ��100g 10%���Ȼ�����Һϡ�ͳ�2%����Ҫ����ˮ������______g��
���𰸡����ƿ �� �ڢ٢ݢۢ� D AD 10 90 400��
��������
��1��ͼ����ʢ���Ȼ��ƹ�������������ǹ��ƿ��������ƽ��ʹ��Ҫ��ѭ���������롱��ԭ��ͼ������ʾ����������ҩƷλ�÷ŷ��ˣ�
��2������100g 10%�Ȼ�����Һ�����ȼ���������Һ�����Ȼ��ƺ�ˮ���������ٳ���������Ȼ��ƺ���ȡˮ���������ܽ⣬������Һ����ȷ����˳��Ϊ�ڢ٢ݢۢܣ�
��3����������=��Һ���������ʵ���������������100g 10%�Ȼ�����Һ�����Ȼ��Ƶ�����=100g��10%=10g���ܼ�����=��Һ����-���������������ܼ�������Ϊ100g-10g=90g����90mL����Ӧ�ù��Ϊ100mL����Ͳ��ȡˮ���������ѡD��
��4��A���Ȼ��ƹ��岻���������ʵ����ȡ�����ʵ�����ƫС����ʹ������������ƫС����A��ȷ��
B����������������������ʵ����ȡ�����ʵ�����ƫ����ʹ������������ƫ��B����
C����Һ���о�һ�ԣ�װƿʱ��������Һ���������������������䣬��C����
D���ܽ�ʱ�ձ��ڱ���ˮ�飬�����ʵ����ȡ��ˮ�����ƫ����ʹ������������ƫС����D��ȷ����ѡAD��
��5������������=��Һ���������ʵ���������������100g 10%���Ȼ�����Һ����Ҫ�Ȼ���=100g��10%=10g���ܼ�����=��Һ����-����������������ˮ������=100g-10g=90g��
������Ҫ����ˮ������Ϊx��
100g��10%=��100g+x����2% x=400g��

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�