��Ŀ����
����Ŀ����λͬѧ�ֱ�����ͬ����������ϡ���ᣬ�ⶨijʯ��ʯ��Ʒ��̼��Ƶ��������� (ʯ��ʯ�е����ʼȲ����ᷴӦ��Ҳ������ˮ)��
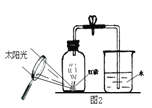
��С���ķ��������������̱�ʾ��
![]()

��.��Ʒ��̼��Ƶ���������Ϊ ��15�� ��
��.ϡ������������������ݷ���ʽ���㣩(16) ����С��ȡ10����Ʒ������ͼʵ��װ�ý���ʵ�顣
����Cװ�õ������� ��17�� ����Ӧ�������Bװ������������4.6�ˣ����ݴ����ݣ�
���̼��Ƶ���������������ʵ���ز����������������Ŀ���ԭ���� ��18�� ��
���𰸡���79%��16��7.3%�����տ����е�CO2��ˮ������Bװ��������CO2������ˮ������HCl����
��������
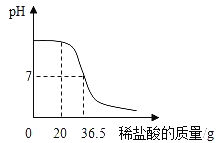
�������: ����ʯ��ʯ�е���Ҫ�ɷ�Ϊ̼��ƣ��������ᷴӦ���ܽ⣻������֪��ʯ��ʯ������Ϊ2.1g����̼�������Ϊ10g-2.1g=7.9g������������Ϊ7.9g/10g��100%=79%��
�� Ӧ�ҳ�������ȫ��Ӧʱ���������������Ȼ����������������ã���֪��50g���ᣬ�Է�Ӧ��̼�������Ϊ10g-5g=5g�����û�ѧ����ʽ��������Ȼ����������
��μӷ�Ӧ��ϡ�������������ΪY����
CaCO3+2HCl=CaCl2+CO2��+H2O
100 73
5g 50gY
100:73==5g:50gY
Y =7.3%
NaOH��������Ϊ�����CO2��������Cװ�õ����������տ����е�CO2��ˮ����������Ӱ��ʵ�������ڷ�Ӧ��ӷ�HCl���壬Ҳ����NaOH��Ӧ����Bװ��������CO2������ˮ������HCl����