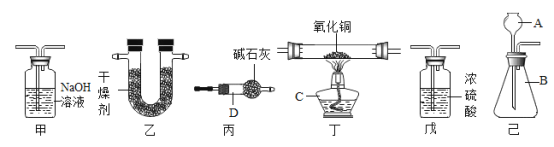
��Ŀ����
����Ŀ���Ķ�������ն��ġ�
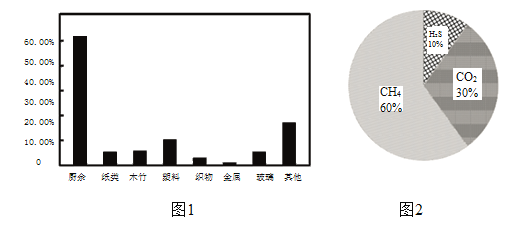
��������ˮƽ����ߣ���������Ҳ���Խ��Խ�ࡣ2018�걱�������������������ռ������ͼ��ʾ��
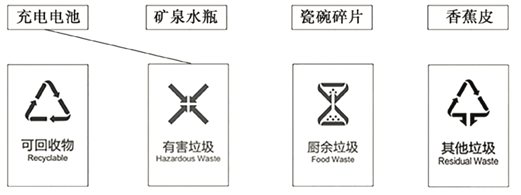
��������������������������������������Ϊ���࣬�����ò�ͬ��ɫ������Ͱ�������֣�������������ɫ�����ɻ������ɫ�����к���������ɫ����������������ɫ����
��� | ��Ҫ���� |
�������� | �˰��Ҷ���Ϲ�Ƥ�ǡ������̡�ʣ��ʣ������Ҷ���� |
�ɻ����� | �ϲ������Ͻ����������ϡ��Ͼ�֯���ֽ�š����鼮�� |
�к����� | ����ء��¶ȼơ�����Һ������ӫ��ƹܡ�����ҩƷ��ɱ����� |
�������� | ����ֽ�����ϴ���ֽ��㡢�ͺС�����ǡ��մ���Ƭ�� |
��������������ڽ���������ϴ�������������Ⱦ���⣬���������������������ԡ����������ͨ���������ͷ�ת��Ϊ��������Ҫ�ɷ���ͼ��ʾ�������ᴿ�������Ƴ�CNGѹ����Ȼ����CNG��CH4������97%��
����������Ҫÿһ���˵�֧������롣
�����������ݣ��ش��������⣺
������������ӦͶ�������Ͱ����_______��
��2����������Ҫ�ɷ�Ϊ���飨CH4����������ȫȼ�յIJ�����_______��
��3�����������������_______��д��һ������
��4����������ת��Ϊ�����ķ�����_______��
��5������˵����ȷ����_______��
A 2018�걱�������������г���������ռ�������
B ��������������Ϊ���࣬�ò�ͬ��ɫ������Ͱ����
C �����Ƴ�CNGѹ����Ȼ������Ҫȥ����H2S��CO2
D �������ж��������ڼ��ٳ�������
���𰸡� CO2��H2O ����������ϴ����������ĵ���Ⱦ���⣬�����������������ԣ����������𰸾��ɣ� �������ͷ� ABCD
CO2��H2O ����������ϴ����������ĵ���Ⱦ���⣬�����������������ԣ����������𰸾��ɣ� �������ͷ� ABCD
��������
��1�����������ṩ�����ϣ�����������к���������Ȫˮƿ���������Ƴɵģ��Ͽ�Ȫˮƿ���ڿɻ����������Ƭ���������������㽶Ƥ���ڳ��������������������������ӦͶ�������Ͱ����Ϊ��

��2�������ڿ�������ȫȼ�����ɶ�����̼��ˮ���ʼ�����ȫȼ�յIJ����ǣ�CO2��H2O��
��3��������������ദ���������������ܴԻ�����Ӱ�����������Ҳ�ܴ�������������һ�𣬻������˴������Ѷȵȡ������������������У�����������ϴ����������ĵ���Ⱦ���⣬�����������������ԣ����������𰸾��ɣ���
��4�����������ṩ����Ϣ����������ת��Ϊ�����ķ����ǣ��������ͷ���
��5��A���������ṩ��2018�걱�������������������ռ����ͼ��֪��2018�걱�������������г���������ռ�����ﵽ50%���ϣ�ѡ��A˵����ȷ��
B����������������������������������������Ϊ���࣬�����ò�ͬ��ɫ������Ͱ�������֣�������������ɫ�����ɻ������ɫ�����к���������ɫ����������������ɫ����ѡ��B˵����ȷ��
C���������ṩ��ͼʾ��֪����������ͨ���������ͷ�ת��Ϊ����������Ҫ�ɷ��У�CH4��H2S��CO2�������ᴿ�������Ƴɵ�CNGѹ����Ȼ����CH4������97%���ɼ��������Ƴ�CNGѹ����Ȼ������Ҫȥ����H2S��CO2��ѡ��C˵����ȷ��
D���������ж���������ʣ��ʣ���������ڼ��ٳ���������ѡ��D˵����ȷ����˵����ȷ���ǣ�ABCD��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ������ʵ����������ﵽʵ��Ŀ�ĵ���
|
|
|
|
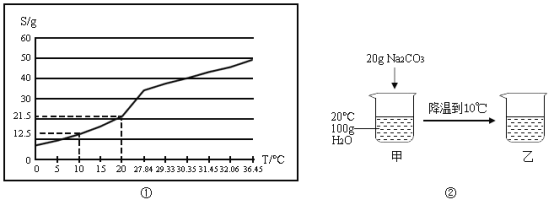
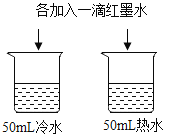
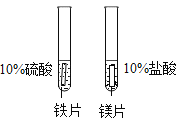
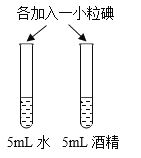
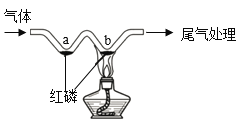
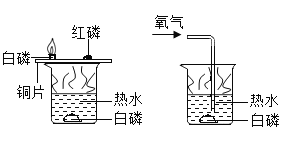
A��̽���¶ȶԷ����˶�������Ӱ�� | B��̽��������̼��ˮ�Ƿ�����Ӧ | C��̽������þ�������ǿ�� | D��̽�������ڲ�ͬ�ܼ����ܽ�������С |
A.AB.BC.CD.D