��Ŀ����
����Ŀ��ij���������ij����̼���Ʋ�Ʒ�п��ܺ����Ȼ������ʡ�
���Լ���
(1)Ҫȷ���ò�Ʒ���Ƿ��������Ȼ��ƣ���ķ�����_________________��
�����ⶨ:
(2)�����ó������ⶨ�ò�Ʒ��̼���Ƶ�������������ȷ���ķ�Ӧԭ����(�û�ѧ����ʽ��ʾ)_______________________________��
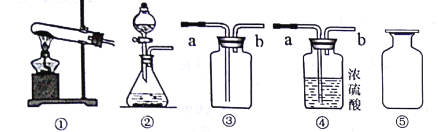
(3)������ȡ��������Ϊm1��������ȷ���ķ�Ӧԭ��������Ҫͨ��ʵ���������(�ѧʽ)____������(�������õij���������Ϊm2)��
(4)�������ʵ�鷽�����������m1��m2���ѷ��������±��С�
���õ��������� | ʵ�鲽�� |
____________ | ______ |
(5)���ݴ�����
���ú�m1��m2��ʽ�ӱ�ʾ������Ʒ��̼���Ƶ�����������______________________��
��������:
(6)Ҫ�ⶨ�������ij�ɷֵĺ�������ͨ����ѧ���������ײⶨ������ת��Ϊ�ײ��������ʡ����������⣬�������ݷ�Ӧ(�û�ѧ����ʽ��ʾ)_______________���ⶨ�ô�����Ʒ��̼���Ƶ�����������
���𰸡� ȡҩƷ���ܽ���ˮ�������������������Һ��Ca(NO3)2 + Na2CO3 == CaCO3��+ 2NaNO3��
���ˣ�����Һ�м�����������Һ���ٵ���ϡ���ᣬ������ְ�ɫ������NaCl+ AgNO3==AgCl��+ NaNO3������Ʒ�л������Ȼ��ơ� Na2CO3+BaCl2=BaCO3��+2NaCl BaCO3 (��CaCO3) �ձ�������������ͷ�ιܡ�����̨(����Ȧ)��©����������ƽ(����ӳ�)��(ϴƿ����������) �ٳ�����Ʒ���������ܽ⣻�ۼ��Լ�(��ָ���Լ�����������)���ܹ��ˢ�ϴ�ӡ������������������ ![]() ��100% Na2CO3 + 2HCl == 2NaCl + H2O + CO2����2NaOH + CO2=== Na2CO3 + H2O
��100% Na2CO3 + 2HCl == 2NaCl + H2O + CO2����2NaOH + CO2=== Na2CO3 + H2O
��������(1)ȡҩƷ���ܽ���ˮ�������������������Һ��Ca(NO3)2 + Na2CO3 == CaCO3��+ 2NaNO3��
���ˣ�����Һ�м�����������Һ���ٵ���ϡ���ᣬ������ְ�ɫ������NaCl+ AgNO3==AgCl��+ NaNO3������Ʒ�л������Ȼ��ơ�(2)�����ó������ⶨ�ò�Ʒ��̼���Ƶ������������ǽ�̼����ת��Ϊ̼�ᱵ��̼��Ƴ�����(3)����������̼��ƣ�CaCO3����̼�ᱵ��BaCO3��������Ȼ���ɳ������������̼���Ƶ����������������Ʒ��̼���Ƶ�����������(4)�ȳ�����Ʒ���������ܽ⡢�μ��������Ȼ�����Һ�����ˡ�ϴ�ӳ�����������������������������������õ���������������ƽ���ձ������������ιܡ�����̨(����Ȧ)��©����ϴƿ����������(5)����Ʒ��̼���Ƶ�������x��BaCl2 + Na2CO3 == BaCO3��+ 2NaCl
106 197![]()
x m2
![]() =
=![]() �����x=
�����x=![]() ����Ʒ��̼���Ƶ����������ǣ�
����Ʒ��̼���Ƶ����������ǣ�![]() ��100%��(6)����Ʒ�м���������ϡ���ᣬ������������ͨ����������������Һ�У�����������Һ���ӵ����������ɶ�����̼��������Ȼ����ݶ�����̼�������̼���Ƶ����������������Ʒ��̼���Ƶ�����������
��100%��(6)����Ʒ�м���������ϡ���ᣬ������������ͨ����������������Һ�У�����������Һ���ӵ����������ɶ�����̼��������Ȼ����ݶ�����̼�������̼���Ƶ����������������Ʒ��̼���Ƶ�����������

 ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д� ��Ԫ����ĩ��ϰ�ȷ��ϵ�д�
��Ԫ����ĩ��ϰ�ȷ��ϵ�д�