��Ŀ����
�������������������й㷺��Ӧ�ã�
��1��������������Ҫ���������������õ� �ԣ�
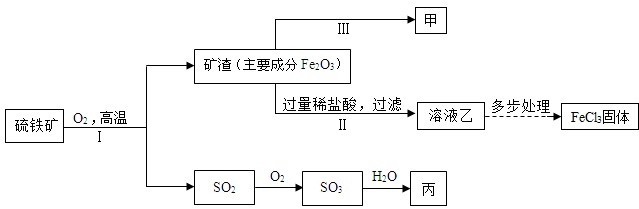
��2����ҵ����һ����̼�ͳ�������Ҫ�ɷ���Fe2O3�����������Ļ�ѧ����ʽΪ ��
��3������ͭ�Ǻ����̡�ͭԪ�صĺϽ𣮽��̣�Mn��Ƭ��������ͭ��Һ�У�һ��ʱ��۲쵽��Ƭ���渽�ź�ɫ���壮����ʵ�������жϣ��̺�ͭ�Ľ��������ǿ������˳���� ��
��4�����������Դ��������������������Դ�Ĵ�ʩ�� ��дһ������
��1��������������Ҫ���������������õ�
��2����ҵ����һ����̼�ͳ�������Ҫ�ɷ���Fe2O3�����������Ļ�ѧ����ʽΪ
��3������ͭ�Ǻ����̡�ͭԪ�صĺϽ𣮽��̣�Mn��Ƭ��������ͭ��Һ�У�һ��ʱ��۲쵽��Ƭ���渽�ź�ɫ���壮����ʵ�������жϣ��̺�ͭ�Ľ��������ǿ������˳����
��4�����������Դ��������������������Դ�Ĵ�ʩ��
��������1���������������������˽����������õĵ����ԣ�
��2�����������Ҫ�ɷ���Fe2O3��Fe2O3���ڸ�������һ����̼��Ӧ��������������������Ҫ�ֶΣ�
��3������������ͭ��Һ�л�ԭ��ͭ��˵���̵Ļ��Ҫ��ͭǿ��
��4���ӽ����ı�������Դ�������÷����������
��2�����������Ҫ�ɷ���Fe2O3��Fe2O3���ڸ�������һ����̼��Ӧ��������������������Ҫ�ֶΣ�
��3������������ͭ��Һ�л�ԭ��ͭ��˵���̵Ļ��Ҫ��ͭǿ��
��4���ӽ����ı�������Դ�������÷����������
����⣺��1���������������������˽����������õĵ����ԣ�
��2�����������Ҫ�ɷ���Fe2O3��Fe2O3���ڸ�������һ����̼��Ӧ��������������������Ҫ�ֶΣ��仯ѧ����ʽ�ǣ�3CO+Fe2O3
2Fe+3CO2��
��3������������ͭ��Һ�л�ԭ��ͭ��˵���̵Ļ��Ҫ��ͭǿ��
��4���ӽ����ı�������Դ�������÷������������ֹ������ʴ����������÷Ͼɽ�����Ѱ�ҽ�������Ʒ���мƻ��������ؿ��ɿ����
�ʴ�Ϊ����1�����ȣ���2��3CO+Fe2O3
2Fe+3CO2����3��Mn��Cu����4����ֹ������ʴ��
��2�����������Ҫ�ɷ���Fe2O3��Fe2O3���ڸ�������һ����̼��Ӧ��������������������Ҫ�ֶΣ��仯ѧ����ʽ�ǣ�3CO+Fe2O3
| ||
��3������������ͭ��Һ�л�ԭ��ͭ��˵���̵Ļ��Ҫ��ͭǿ��
��4���ӽ����ı�������Դ�������÷������������ֹ������ʴ����������÷Ͼɽ�����Ѱ�ҽ�������Ʒ���мƻ��������ؿ��ɿ����
�ʴ�Ϊ����1�����ȣ���2��3CO+Fe2O3
| ||
������������Ҫ��������ĸ������ʣ�ֻҪ��֪������ݣ�����ʵ�鼰����˵������ϸ�µķ������ɵ���ȷ�𰸣�

��ϰ��ϵ�д�
�����Ŀ

 ��2013?��������ģ���������������������й㷺��Ӧ�ã�
��2013?��������ģ���������������������й㷺��Ӧ�ã� ��2013?��������ģ���������������������й㷺��Ӧ�ã�
��2013?��������ģ���������������������й㷺��Ӧ�ã�