��Ŀ����
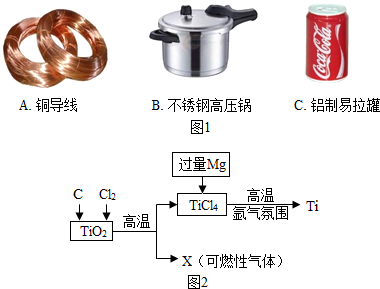 ��2013?��������ģ���������������������й㷺��Ӧ�ã�
��2013?��������ģ���������������������й㷺��Ӧ�ã���1����������ͭ����������������ʹ�ñȽϹ㷺�Ľ�����ͼ1��Ʒ�У���Ҫ���ý��������Ե���
B
B
������ţ�����2�������ѣ�Ti����Ϊ�����������Խ��Խ�������ǵĹ�ע�����Ǻ��ա��������������������Ҫ��ԭ���ϣ�Ŀǰ���ģ�����ѵ�������ͼ2��ʾ��
��ʹTiO2��C��ϣ��ڸ��������·�Ӧ����ͨ��Cl2���Ƶ�TiCl4��һ�ֿ�ȼ������X������ݷ�Ӧ�������ǰ��ı仯���Ʋ�X������Ϊ��
һ����̼
һ����̼
����������������У��������ù�����Mg��TiCl4�����û���Ӧ�Ƶ��ѣ�д���˷�Ӧ�Ļ�ѧ����ʽ��
2Mg+TiCl4
Ti+2MgCl2
| ||
2Mg+TiCl4
Ti+2MgCl2
���ù�������������ã�
| ||
������������ֹ����������Ӧ
������������ֹ����������Ӧ
����3���ò�����п��ϡ���ᷴӦ�ܼӿ�������������ʣ�ʵ������13g п�ۺ�2g ͭ�۵Ļ������������ϡ���ᷴӦ�������ɶ��ٿ���������д��������̣�
��������1�������������õĵ����ԡ������ԡ���չ�ԣ�
��2�����������غ㶨�ɺ���ط������Ϣ�����ж����ʵ����ƣ����ݷ�Ӧ��������Ӧ�������������غ㶨�ɿ�����д��ѧ����ʽ��
��3�����ݻ�ѧ����ʽ���Լ�������������������
��2�����������غ㶨�ɺ���ط������Ϣ�����ж����ʵ����ƣ����ݷ�Ӧ��������Ӧ�������������غ㶨�ɿ�����д��ѧ����ʽ��
��3�����ݻ�ѧ����ʽ���Լ�������������������
����⣺��1���������������--��ѹ�����������˲���־������õĵ����ԣ�
���B��
��2����TiO2��C��ϣ��ڸ��������·�Ӧ����ͨ��Cl2��������TiCl4�⣬���ܹ�����һ����̼���壮
���һ����̼��
��Mg��TiCl4�ڸ��������·�Ӧ��������Ti��MgCl2����ѧ����ʽΪ��2Mg+TiCl4
Ti+2MgCl2��
���2Mg+TiCl4
Ti+2MgCl2��
����Ļ�ѧ���ʺܲ����ã�����������ȡ�����ѵı�������
���������������ֹ����������Ӧ��
��3���⣺��������������Ϊx��
ͭ���������ᷴӦ���ܲ���������ֻ��п����ϡ����ķ�Ӧ��
Zn+H2SO4�TZnSO4+H2����
65 2
13g x
=
��
x=0.4g��
�𣺿���������0.4g��
���B��
��2����TiO2��C��ϣ��ڸ��������·�Ӧ����ͨ��Cl2��������TiCl4�⣬���ܹ�����һ����̼���壮
���һ����̼��
��Mg��TiCl4�ڸ��������·�Ӧ��������Ti��MgCl2����ѧ����ʽΪ��2Mg+TiCl4
| ||
���2Mg+TiCl4
| ||
����Ļ�ѧ���ʺܲ����ã�����������ȡ�����ѵı�������
���������������ֹ����������Ӧ��
��3���⣺��������������Ϊx��
ͭ���������ᷴӦ���ܲ���������ֻ��п����ϡ����ķ�Ӧ��
Zn+H2SO4�TZnSO4+H2����
65 2
13g x
| 65 |
| 2 |
| 13g |
| x |
x=0.4g��
�𣺿���������0.4g��
������������Ҫ�������ʵ����ʺ���;����ѧ����ʽ����д�����ݻ�ѧ����ʽ����ȷ����֪ʶ�����ݻ�ѧ����ʽ����Ƚϼ�����ʱֻҪע��淶�Ա��˳�����

��ϰ��ϵ�д�
 ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д� �����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д�
�����ѧСѧ�꼶�νӵ������㽭��ѧ������ϵ�д� Сѧ�����ҵ���ϴ�ѧ������ϵ�д�
Сѧ�����ҵ���ϴ�ѧ������ϵ�д� ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д�
�����Ŀ