��Ŀ����
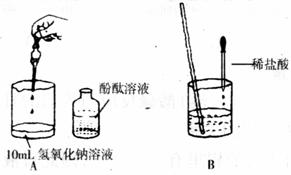
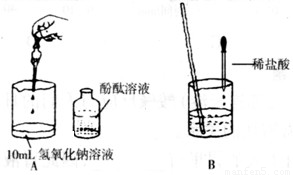
��1������������һ�ֳ��õļ����Һ�Լ��ԣ�ԭ���Ǹ���Һ�к���______���ӣ�д���ӷ��ţ�������Һ��pH______7�����������=��������2������ͬѧ������ͼ������������������Һ��ϡ���ᷢ������кͷ�Ӧ��ʵ�飮���μӷ�̪����Һ��______ɫ������ٵμ�ϡ����ʱ�����������μ��ˣ���ѡ��______��дһ���Լ����ƣ���֤�����������
��3������λͬѧҲչ����̽������С��ȡ��һƿ����������Һ������û���ܷ�ã��⽫�����Һ���տ����е�______�����ʣ��ݴ�����������һ�����������______��С����̽�����Ƿ���ʣ�����С�Թ�ȡ����Һ������Ȼ��μӼ�����ɫ��̪����Һ��______ɫ���������жϸ���Һû�б��ʣ������ж���ȷ��______�����ȷ������ȷ������
��С����С�Թ�ȡ��Һ������Ȼ����εμ�ϡ���ᣬ��ʼ���Σ���û�з������ݷų������������μ�ʱ���������ݣ�����������Ϳ�ʼû�����ݷų���ԭ��______��
��4��ȡ15���Ѳ��ֱ��ʵ�NaOH���壬���뵽50��ϡ�����У���ַ�Ӧ����ʣ��������ֻ��60.6�ˣ���ù����к��������Ƶ�������
���𰸡���������1�����ݼ����ɣ���Һ�������pH�Ĺ�ϵ����
��2��������Ļ�ѧ���ʷ���
��3���������������ܷⱣ���ԭ�����
��4�����ݶ�����̼�����������̼�����������Ӷ�����������Ƶ�������
����⣺��1������������һ�ֳ��õļ����Һ�Լ��ԣ�ԭ���Ǹ���Һ�к��� OH-���ӣ�����Һ��pH��7��
��2������������Һ�Լ��ԣ���ʹ��̪��Һ��죬��������ý�����̼���η�Ӧ�������ݣ�
��3��������������������еĶ�����̼��Ӧ������̼���ƶ����ʣ������ʹ������������ҺʱҪע�⣺�����Լ������ָ���ƿ������Ϊ̼������Һ�ʼ��ԣ�Ҳ��ʹ��̪��Һ��죬���С�췽������ȷ��
�ڿ�ʼʱ��Һ�к����������ƣ��������ᣬ�������������ᷴӦ��û���������ɣ�������������ȫ�кͺ�̼���������ᷴӦ�������ݣ�
��4����Ӧǰ���������ļ�������Ϊ���ɵĶ�����̼����������15g+50g-60.6g=4.4g
��μӷ�Ӧ��Na2CO3����Ϊx
Na2CO3+2HCl�T2NaCl+CO2+2H2O
106 44
x 4.4
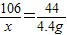
x=10.6g
��������������Ƶ�����Ϊ15g-10.6g=4.4g��
�𣺸ù����к��������Ƶ�����Ϊ4.4g��
�ʴ�Ϊ����1��OH-��
��2���� ̼���ƣ���п�������û��ý�������
��3����CO2 ʵ��ʱ�����Լ������ָ���ƿ�� �� ����ȷ �ڿ�ʼʱ��Һ�к����������ƻ���������û�б��к���
��4��4.4g��
���������⿼������кͷ�Ӧ���������Ʊ���ԭ��̼���Ƶ���֤ʵ����ƣ�����ѧ���������⣬������֪֪ʶ��������������
��2��������Ļ�ѧ���ʷ���
��3���������������ܷⱣ���ԭ�����
��4�����ݶ�����̼�����������̼�����������Ӷ�����������Ƶ�������
����⣺��1������������һ�ֳ��õļ����Һ�Լ��ԣ�ԭ���Ǹ���Һ�к��� OH-���ӣ�����Һ��pH��7��
��2������������Һ�Լ��ԣ���ʹ��̪��Һ��죬��������ý�����̼���η�Ӧ�������ݣ�
��3��������������������еĶ�����̼��Ӧ������̼���ƶ����ʣ������ʹ������������ҺʱҪע�⣺�����Լ������ָ���ƿ������Ϊ̼������Һ�ʼ��ԣ�Ҳ��ʹ��̪��Һ��죬���С�췽������ȷ��
�ڿ�ʼʱ��Һ�к����������ƣ��������ᣬ�������������ᷴӦ��û���������ɣ�������������ȫ�кͺ�̼���������ᷴӦ�������ݣ�
��4����Ӧǰ���������ļ�������Ϊ���ɵĶ�����̼����������15g+50g-60.6g=4.4g
��μӷ�Ӧ��Na2CO3����Ϊx
Na2CO3+2HCl�T2NaCl+CO2+2H2O
106 44
x 4.4
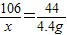
x=10.6g
��������������Ƶ�����Ϊ15g-10.6g=4.4g��
�𣺸ù����к��������Ƶ�����Ϊ4.4g��
�ʴ�Ϊ����1��OH-��
��2���� ̼���ƣ���п�������û��ý�������
��3����CO2 ʵ��ʱ�����Լ������ָ���ƿ�� �� ����ȷ �ڿ�ʼʱ��Һ�к����������ƻ���������û�б��к���
��4��4.4g��
���������⿼������кͷ�Ӧ���������Ʊ���ԭ��̼���Ƶ���֤ʵ����ƣ�����ѧ���������⣬������֪֪ʶ��������������

��ϰ��ϵ�д�
�����Ŀ
 ������������⣬д�������һ����������
������������⣬д�������һ���������� ����һ������ѧ������ˮ���ռ��õ�һƿ��ɫ���壬����ƿ�е����������
����һ������ѧ������ˮ���ռ��õ�һƿ��ɫ���壬����ƿ�е���������� ��1������������һ�ֳ��õļ����Һ�Լ��ԣ�ԭ���Ǹ���Һ�к���
��1������������һ�ֳ��õļ����Һ�Լ��ԣ�ԭ���Ǹ���Һ�к���