��Ŀ����
����Ŀ��ʳ�����������Ʒ������Ҫ�Ļ���ԭ�ϣ�Ҳ��ͬѧ��ʵ��ʱ���õ�ҩƷ��
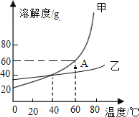
(1)ʳ���к��и������Ӽ��������ӵ����(KIO3)�����Ԫ�ء����������軯��(K4[Fe(CN)6]3H2O)��Ϊ�������ֹʳ�ν�顣
��ʳ�������ٺ���_____________�ֽ���Ԫ��(������)��
�������軯���еĽ��������� K+��____________(��������)��
(2)��ⱥ��ʳ��ˮ���Եõ����ֻ�����Ʒ����Ҫ�������£�
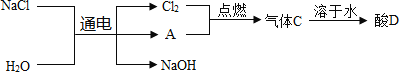
��������A ��һ�ֵ��ʣ��������Ϊ__________��������������_____________��
�ڵ�������������ƵĻ�ѧ����ʽΪ_____________��
����D��Һ�����ʵĻ�ѧʽΪ_________________��
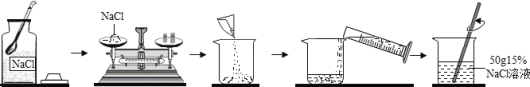
(3)��ͼ��ʵ��������һ�����������������Ȼ�����Һ������ͼ��
��ش�
��Ӧ����____________g�Ȼ��ƹ��壬���ˮ_____________mL��
���������Ȼ��ƹ���ʱָ������ƫת����Ӧ______________ֱ����ƽƽ�⡣
��ʵ���з����Ȼ��ƹ����ѽ�飬Ϊ�˽Ͽ���ܽ⣬�����ķ�����_____________(�����)��
A���ò��������Ȼ��ƹ��嵷�� B������ˮ�ܽ�
C����������ˮ D���ò���������
�����в����п��ܵ��������Ȼ�����Һ��������������ƫС����___________(�����)��
A���Ȼ����к������� B����ȡˮʱ����
C������������ˮ���ձ��ܽ��Ȼ��� D��ת��ʱ����������Һ����
��ȡ������Һ10g�����______________gˮϡ�ͣ��ɵõ�5%���Ȼ�����Һ��
���𰸡�3 Fe2+ H2 ��Ӧǰ��Ԫ�������غ�(�����غ㶨�ɵ�) 2NaCl+2H2O![]() 2NaOH+H2��+Cl2�� HCl 7.5g 42.5mL �����̼������Ȼ��� BD ABC 20g
2NaOH+H2��+Cl2�� HCl 7.5g 42.5mL �����̼������Ȼ��� BD ABC 20g
��������
(1)��ʳ���к��и������Ӽ��������ӵ���أ�KIO3�������Ԫ�ء����������軯�أ�K4[Fe��CN��6]3H2O��������ʳ���к��еĽ���Ԫ������Ԫ�ء���Ԫ�ء���Ԫ�أ�
�ڸ��������軯�أ�K4[Fe��CN��6]3H2O������֪���еĽ��������� K+��Fe2+ ��
(2)�ٸ��������غ㶨�ɿ�֪����Ӧǰ��Ԫ������䣬����A ��������
�ڵ�ⱥ��ʳ��ˮ�Ļ�ѧ����ʽΪ��2NaCl+2H2O![]() 2NaOH+H2��+Cl2����
2NaOH+H2��+Cl2����
��������������ȼ�����Ȼ������壬����ˮ�������ᣬ���ʵĻ�ѧʽΪHCl��
(3)����������=��Һ���������ʵ���������������15%���Ȼ�����Һ50g�����Ȼ��Ƶ�����=50g��15%=7.5g���ܼ�����=��Һ����-����������������ˮ������=50g-7.5g=42.5g����42.5mL����
��������NaCl����ʱ����Ӧ���ǣ��ȵ�����������룬Ȼ���������������ʳ�Σ��ڳ����з���ָ������ƫת��˵����������ҩƷ������С���������������Ӧ���������������Ȼ��ƣ�ֱ����ƽƽ�⣻
�����Ȼ��ƹ����ѽ�飬Ϊ�˽Ͽ���ܽ⣬���Խ��Ȼ������飬������Ӵ����������ˮ�ܽ⡢�ò��������裬�Լӿ��ܽ����ʣ�
��A���Ȼ����к������ʣ������ܽ��Ȼ�������ƫС���������Ȼ�����Һ����������ƫС������ȷ��
B����ȡˮʱ���ӣ�����С������ȡˮ��ʵ�������ʹ��ȡˮ�����ƫ���������Ȼ�����Һ����������ƫС������ȷ��
C���ձ��ڱ��ϲ�������ϴ�ձ����µ�����ˮ��ˮ�������˶�ƫ���������Ȼ�����Һ����������ƫС������ȷ��
D�����ƺõ���Һ����ϸ��ƿʱ����ƿ�⣬������ɵ���Һ���䲻Ӱ����Һ�����������������������䣬����ȷ��
����Ҫ��ˮ������Ϊx��������Һϡ��ǰ�����ʵ��������䣬��l0g��15%=��10g+x����5%��x=20g��

����Ŀ��ij��ѧ��ȤС����ʵ������ģ�����������ǰѽ�̿����������ĩ��ϼ�ǿ�ȣ��õ���ɫ����A�ͺ�ɫ��ĩB(��ͼ��ʾ)��
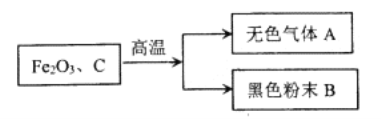
(1)����������Ӧ�Ļ�ѧ����ʽ��__________��
(2)��������֪����ɫ����A�����е��ʣ���ɫ��ĩB�ijɷ���һ�ֻ����ֵ��ʡ��ס���������ȤС��ֱ����ɫ����A�ͺ�ɫ��ĩB�ijɷֽ���̽����
����������裩������Ϊ��ɫ����A�п�����CO2��Ҳ������CO��
������Ϊ��ɫ��ĩ�ijɷ��д������������������ֻ����______���ں�������̼��
��ʵ��̽����(1)Ϊ��֤����IJ��룬ͬѧ�ǰ���ͼ��ʾװ�ý���ʵ�顣
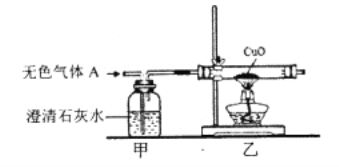
װ�ü�������_______��װ���ҵ�������_______�����������г���ʯ��ˮ����ǣ�����____����ȷ����ɫ����A��CO2��CO�Ļ�����塣
��ͬѧ����Ѽס��ҽ���λ�ã�������A��ͨ���ȵ�����ͭҲ���Դﵽͬ����ʵ��Ŀ�ģ�����ж���_____(����������������������)��
(2)Ϊ��֤�����м�����Ƿ���������ʵ���������ͻ�ѧ���ַ����������̽����
ʵ�鷽�� | ʵ����� | ʵ������ | ʵ����� |
�������� | _______ | _______ | ��������� |
��ѧ���� | ______ | ���ֺ�ɫ��ĩ�ܽ⣬������ɫ���� | ��������� |
��������˼����Լ���ʵ����ڵ�ȱ�ݣ�ͬѧ��һ������˸Ľ����������Ⱦ�������⡣
����Ŀ��������ҹ���������ʳ����ʷ�ƾá�������õ�һ��ȼ���ǹ���ƾ���ij��ѧ��ȤС���ͬѧ��������ƾ��������˺��棬����ɷֽ���̽��������ش��������⡣
���������ϣ�
a������ƾ�Ҳ����Ϊ"�ƾ���"�����ȼ�Ͽ顣����ƾ������ǹ���״̬�ľƾ����ǽ��ƾ���Ӳ֬����������ư�һ���������Ȼ���Ƴɡ�
b���ƾ��Ļ�ѧʽΪC2H5OH��
c.�Ȼ������Ȼ�����Һ�������ԡ�
d. BaCl2+Na2CO3=BaCO3��+2NaCl ���ɵ�BaCO3Ϊ��ɫ����
��������⣩
(1)�ƾ��Ļ�ѧʽ��NaOH��ȣ�������OH������ô�ƾ���ˮ��Һ�Dz����Լ��ԣ�
(2)����ƾ��е����������Ƿ���ʼ����ʵij̶���Σ�
��ʵ��̽��1���ƾ���ˮ��Һ�Dz����Լ���
ͬѧ��ȡ�����ƾ���Һ���Թ��У��μ���ɫʯ����Һ��δ�۲쵽��ɫʯ���Ϊ��ɫ��˵���ƾ���Һ_______(������������������)���ԡ�
��ʵ��̽��2������ƾ��е����������Ƿ���ʼ����ʵij̶����
�ٹ���ƾ��е����������Ƿ���ʣ�ͬѧ����ȡ��������ƾ����ձ��У���������ˮ�ܽ��μ�������ϡ���ᣬ�۲쵽__________����˵�����������ѱ��ʣ���д�����������ڿ����б��ʵĻ�ѧ����ʽ______________��
��Ϊ��һ��ȷ���������Ƶı��ʳ̶ȣ��������̽����
����ͬѧȡ�ձ��ϲ���Һ����֧�Թ��У�����ͼ��ʾ����ʵ�顣
ʵ�鷽�� |
|
|
ʵ������ | ��Һ��� | ����__ |
ʵ����� | ��Һ������������ | ��Һ����̼���� |
����ͬѧ��Ϊ����ʵ�鲻��֤����Һ��һ�����������ƣ�������__��������ȡ�ձ����ϲ���Һ���������Ȼ�����Һ����ַ�Ӧ���ã�ȡ�ϲ���Һ���μӷ�̪��Һ����̪��Һ��졣
����˼����������ʵ���м������Ȼ�����Һ��Ŀ����________��
��ʵ�����]С��ͬѧ�������ۣ�һ����Ϊ�ù���ƾ��е��������Ʋ��ֱ��ʡ�