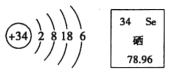
��Ŀ����
����Ŀ��ˮ����Ҫ����Ȼ��Դ��������������ˮ��Դ���ƽ���ᷢչ�������
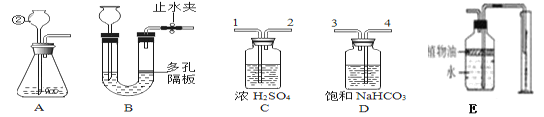
��1����Ȼ���е�ˮ���н϶����ʣ����������м������̿����������______�ԡ�
��2����Ȼˮ����һ����Ӳ�ȣ��ճ�����Ӳˮ����ˮ��ѡ�õ�������______��
��3��ˮ��Ϊԭ�ϣ��ɻ�ö��ֲ�Ʒ��
�ٹ�ҵ�ϳ���ˮ��ȡˮú����ԭ��Ϊ��H2O+C![]() CO+H2���÷�Ӧ�����У�����ת��Ϊ______��
CO+H2���÷�Ӧ�����У�����ת��Ϊ______��
������CO��H2��ȡ�����ѵ��۹�����ͼ��ʾ��
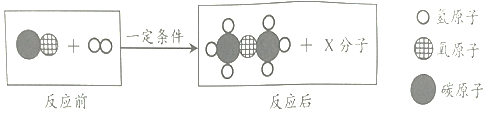
�ñ仯�����У��μӷ�Ӧ��CO��H2�ķ��Ӹ�������______��
���𰸡����� ����ˮ ��ѧ 1��2
��������
��1������̿���������ԣ���������ˮ�е�ɫ�غ���ζ�����������
��2������Ӳˮ��ˮ��ѡ�õ������Ƿ���ˮ���������ˮ����������ĭ�϶࣬������ˮ����������ĭ���٣�����Ӳˮ���������ˮ��
��3���ٹ�ҵ�ϳ���ˮ��ȡˮú����ԭ��Ϊ��H2O+C![]() CO+H2���÷�Ӧ�����У�����ת��Ϊ��ѧ�ܣ������ѧ��
CO+H2���÷�Ӧ�����У�����ת��Ϊ��ѧ�ܣ������ѧ��
���������غ㶨�ɿ�֪��CO��H2��һ�������·�Ӧ���ɶ����Ѻ�ˮ����ѧ����ʽΪ�� ���ñ仯�����У��μӷ�Ӧ��CO��H2�ķ��Ӹ�������2��4=1��2�����1��2��
���ñ仯�����У��μӷ�Ӧ��CO��H2�ķ��Ӹ�������2��4=1��2�����1��2��

��ϰ��ϵ�д�
 �����Ծ���Ԫ���Ծ�ϵ�д�
�����Ծ���Ԫ���Ծ�ϵ�д�
�����Ŀ