��Ŀ����
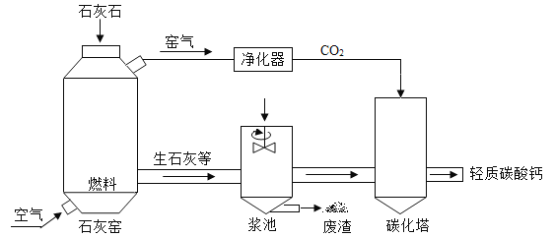
����Ŀ����ҵ����ʯ��ʯ������Ϊԭ���Ʊ�����̼��ƣ���Ҫ�������£�
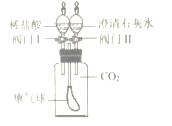
��֪��ʯ��Ҥ�е�ʯ��ʯ������ʯ�ҵķ�ӦΪ��CaCO3![]() CaO+CO2��
CaO+CO2��
��1��ʯ��Ҥ��ʯ��ʯ�����ķ�Ӧ���ڻ�ѧ������Ӧ�����е�_____��Ӧ��
��2�������з�����ӦΪ���Ϸ�Ӧ���仯ѧ����ʽΪ_____��
��3������˵����ȷ����_____������ţ���
A ʯ��Ҥ������Ҥ���Ǵ�����
B ���ص�����֮һ�Ƿ�����ʯ���е�����
C �õ�������̼��Ʊ�ʯ��ʯ��̼��ƵĴ��ȸ�
���𰸡��ֽ� CaO+H2O=Ca��OH��2 BC
��������
��1��ʯ��Ҥ��ʯ��ʯ�ڸ������������������ƺͶ�����̼���÷�Ӧ��������һ��࣬���ڷֽⷴӦ������ֽ⡣
��2����������ʯ�Һ�ˮ��Ӧ�����������ƣ��仯ѧ����ʽΪ��CaO+H2O=Ca��OH��2�����CaO+H2O=Ca��OH��2��
��3��A��Ҥ���к��е�����������������̼�ȣ����ڻ�����ѡ��˵������
B���������ܷ�������������ص�����֮һ�Ƿ�����ʯ���е����ʣ���ѡ��˵����ȷ��
C��ʯ��ʯ����Ҫ�ɷ���̼��ƣ��������ʣ��õ�������̼��Ʊ�ʯ��ʯ��̼��ƵĴ��ȸߣ���ѡ��˵����ȷ��
��ѡ��BC��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ