��Ŀ����
��6�֣�ij��ѧ��ȤС�飬��һ�β��� �����������������θ����ࡱ�����Ϻ�������ʵ���������������������������С�մ�ˮ��NaHCO3ˮ��Һ������������θ������֢״���������Ʋ��裨��ҽ�����飩���£�
��һ����ȷ����8.4gʳƷ��С�մ��ĩ���ڶ���������һ�����Ʒ�ĩ���100g��Һ��
�����������ڶ���������Һȡ��10g���ټ�ˮ���Ƴ�100g��Һ���õ�����θ������
С�մ�ˮ���ܶ�Ϊ1.0g/mL����
��ش��������⣺
��1����һ���õ�����Ҫ������ ���ڶ���ʹ�õIJ��������� ������д���֣�����2���������������õ�С�մ�ˮ��NaHCO3������������ ��
��3��θ���������ҽ����ָ���£�ÿ�κ�50mL�������������õ�С�մ�ˮ��һ�����Σ���
һ��ɷ�Ӧ��θҺ�е�HCl g��
��һ����ȷ����8.4gʳƷ��С�մ��ĩ���ڶ���������һ�����Ʒ�ĩ���100g��Һ��
�����������ڶ���������Һȡ��10g���ټ�ˮ���Ƴ�100g��Һ���õ�����θ������
С�մ�ˮ���ܶ�Ϊ1.0g/mL����
��ش��������⣺
��1����һ���õ�����Ҫ������ ���ڶ���ʹ�õIJ��������� ������д���֣�����2���������������õ�С�մ�ˮ��NaHCO3������������ ��
��3��θ���������ҽ����ָ���£�ÿ�κ�50mL�������������õ�С�մ�ˮ��һ�����Σ���
һ��ɷ�Ӧ��θҺ�е�HCl g��
��6�֣���1��������ƽ����ƽ�� (1��) ���������ձ�����Ͳ����д2�־��ɣ���д��д�����÷֣�1�֣�
��2��0.84%��0.0084�� ��2�֣� ��3��0.37��0.365�� ��2�֣���
��2��0.84%��0.0084�� ��2�֣� ��3��0.37��0.365�� ��2�֣���
������1��������Һ������ѡ������������
��2���������ʵ������������㹫ʽ���н��
��3������С�մ���ϡ���ᷴӦ�Ļ�ѧ����ʽ���
��𣺽⣺��1������Ҫ�õ�������ƽ��������Һ��Ҫ�õ��ձ��������ܽ�IJ���������ȡһ����Һ�����Ͳ��
�ʴ�Ϊ��������ƽ�� ���������ձ�����Ͳ����д2�ּ��ɣ���
��2���ڶ���������Һ��������������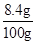 ��100%=8.4%��С�մ�Ϊ10g��8.4%=0.84g����Һ��100g�����ʵ���������
��100%=8.4%��С�մ�Ϊ10g��8.4%=0.84g����Һ��100g�����ʵ���������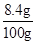 ��100%=0.84%��
��100%=0.84%��
�ʴ�Ϊ��0.84%��
��3��ÿ�������С�մ������50mL��2��1.0g/mL��0.84%=0.84g��
����ɷ�Ӧ��θҺ�е�HCl ������ΪX��
NaHCO3+HCl=NaCl+H2O+CO2��
84 36.5
0.84g X
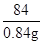 =
=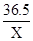
X=0.365g
�ʴ�Ϊ��0.365g��
������������С�մ�����θ�����Ϊ�������ʵ��̽������Һ�������õ����������ʵ������������㣬���ݷ���ʽ�ļ��㣬�����ӱ��������ѧ����Ӧ����������ᵽѧϰ��ѧ��ʵ�����壮
��2���������ʵ������������㹫ʽ���н��
��3������С�մ���ϡ���ᷴӦ�Ļ�ѧ����ʽ���
��𣺽⣺��1������Ҫ�õ�������ƽ��������Һ��Ҫ�õ��ձ��������ܽ�IJ���������ȡһ����Һ�����Ͳ��
�ʴ�Ϊ��������ƽ�� ���������ձ�����Ͳ����д2�ּ��ɣ���
��2���ڶ���������Һ��������������
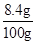 ��100%=8.4%��С�մ�Ϊ10g��8.4%=0.84g����Һ��100g�����ʵ���������
��100%=8.4%��С�մ�Ϊ10g��8.4%=0.84g����Һ��100g�����ʵ���������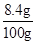 ��100%=0.84%��
��100%=0.84%���ʴ�Ϊ��0.84%��
��3��ÿ�������С�մ������50mL��2��1.0g/mL��0.84%=0.84g��
����ɷ�Ӧ��θҺ�е�HCl ������ΪX��
NaHCO3+HCl=NaCl+H2O+CO2��
84 36.5
0.84g X
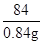 =
=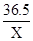
X=0.365g
�ʴ�Ϊ��0.365g��
������������С�մ�����θ�����Ϊ�������ʵ��̽������Һ�������õ����������ʵ������������㣬���ݷ���ʽ�ļ��㣬�����ӱ��������ѧ����Ӧ����������ᵽѧϰ��ѧ��ʵ�����壮

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ