��Ŀ����
С���ڻ�ѧʵ���ҷ��֣�ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ��С�ս���С����С�죬��ͬ̽�����ְ�ɫ��ĩ�ijɷ֡�����������ѧ�Ļ�ѧ֪ʶ�������ְ�ɫ��ĩ�ijɷ��������²���
�� ������NaOH�� �� ������Na2CO3�� �� ������NaOH��Na2CO3�Ļ���Ϊ����֤���룬���Ƿֱ�
���������ʵ�顣
��1��С��ȡ������ɫ��ĩ����ˮ����������Һ�еμӷ�̪��Һ����Һ��Ϊ��ɫ���ɴ�С����Ϊ��ɫ��
ĩ��NaOH�����ж�С�����ý����Ƿ���ȷ�����������ɡ�
_______��____________________________________________________________________��
��2��С��ȡ������ɫ��ĩ����ˮ����������Һ�еμ�BaCl2��Һ���а�ɫ�����������ɴ��жϰ�ɫ��ĩ
�к���______��Ϊ����֤����ۣ�С���������Һ�еμ�BaCl2��Һ�����ٲ���������Ȼ����ˡ�����
Ϊ����������Ӧ���е�ʵ����_____________________________________________����С��������ʵ���У�����BaCl2��Һ����Ba(OH)2��Һ�Ƿ���У���������ɡ�_________��_________________________
______________________________��
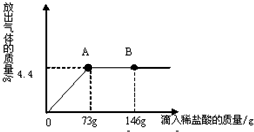
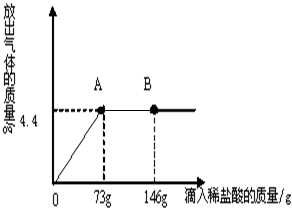
��3����һ�ձ���ʢ��22.3g Na2CO3��NaCl��ɵĹ��������43.1gˮ�ܽ⣬�Ƴ���Һ���������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺��H-1��Cl-35.5��Na-23��C-12��O-16��
�� ������NaOH�� �� ������Na2CO3�� �� ������NaOH��Na2CO3�Ļ���Ϊ����֤���룬���Ƿֱ�
���������ʵ�顣
��1��С��ȡ������ɫ��ĩ����ˮ����������Һ�еμӷ�̪��Һ����Һ��Ϊ��ɫ���ɴ�С����Ϊ��ɫ��
ĩ��NaOH�����ж�С�����ý����Ƿ���ȷ�����������ɡ�
_______��____________________________________________________________________��
��2��С��ȡ������ɫ��ĩ����ˮ����������Һ�еμ�BaCl2��Һ���а�ɫ�����������ɴ��жϰ�ɫ��ĩ
�к���______��Ϊ����֤����ۣ�С���������Һ�еμ�BaCl2��Һ�����ٲ���������Ȼ����ˡ�����
Ϊ����������Ӧ���е�ʵ����_____________________________________________����С��������ʵ���У�����BaCl2��Һ����Ba(OH)2��Һ�Ƿ���У���������ɡ�_________��_________________________
______________________________��
��3����һ�ձ���ʢ��22.3g Na2CO3��NaCl��ɵĹ��������43.1gˮ�ܽ⣬�Ƴ���Һ���������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ�����������ش����⣺��H-1��Cl-35.5��Na-23��C-12��O-16��

�ٵ��μ���73gϡ����ʱ���ų����������Ϊ g�����μ�ϡ������ͼ��B��ʱ���ձ�����Һ��������ǣ�д��ѧʽ�� ��
�ڵ��μ���73gϡ����ʱ����A��ʱ�����ձ���Ϊ��������Һ����ͨ������������к����ʵ�����������[����������һλС��]
�ڵ��μ���73gϡ����ʱ����A��ʱ�����ձ���Ϊ��������Һ����ͨ������������к����ʵ�����������[����������һλС��]
��1������ȷ����ΪNa2CO3��ˮ��ҺҲ�Լ���
��2��Na2CO3��ȡ�ϲ���Һ��������μ���ɫ��̪��Һ���۲������У���Ϊ�绻��Ba(OH)2��Һ���ͼ����Ƿ���NaOH��
��3����4.4g��NaCl��HCl
�ڽ⣺�跴Ӧ������NaCl������Ϊx���������Na2CO3������Ϊy
Na2CO3 + 2HCl ====2NaCl + H2O + CO2��
106 117 44
y x 4.4g
117/44 =x/4.4g x=11.7g
106/44 =y/4.4g y=10.6g
��Ӧ������NaCl����������:[11.7g+(22.3g-10.6g)]/(22.3g+43.1g+73g-4.4g)��100%=17.5%
��2��Na2CO3��ȡ�ϲ���Һ��������μ���ɫ��̪��Һ���۲������У���Ϊ�绻��Ba(OH)2��Һ���ͼ����Ƿ���NaOH��
��3����4.4g��NaCl��HCl
�ڽ⣺�跴Ӧ������NaCl������Ϊx���������Na2CO3������Ϊy
Na2CO3 + 2HCl ====2NaCl + H2O + CO2��
106 117 44
y x 4.4g
117/44 =x/4.4g x=11.7g
106/44 =y/4.4g y=10.6g
��Ӧ������NaCl����������:[11.7g+(22.3g-10.6g)]/(22.3g+43.1g+73g-4.4g)��100%=17.5%

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
 С���ڻ�ѧʵ���ҷ��֣�ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ��С�ս���С����С�죬��ͬ̽�����ְ�ɫ��ĩ�ijɷ֣�
С���ڻ�ѧʵ���ҷ��֣�ʢ��NaOH��Һ���Լ�ƿƿ�ں���Ƥ���ϳ����˰�ɫ��ĩ��С�ս���С����С�죬��ͬ̽�����ְ�ɫ��ĩ�ijɷ֣�