��Ŀ����
 ��2011?���֣�ͬѧ����Na2CO3��Һ��ŨHCl���о�����������ķ�Ӧԭ��ʱ���Է�Һ�ijɷֽ���̽����
��2011?���֣�ͬѧ����Na2CO3��Һ��ŨHCl���о�����������ķ�Ӧԭ��ʱ���Է�Һ�ijɷֽ���̽����[��������]
�����������ʷ�����Ӧ�Ļ�ѧ����ʽΪ
Na2CO3+2HCl=2NaCl+H2O+CO2��
Na2CO3+2HCl=2NaCl+H2O+CO2��
���ɴ��Ʋ����Һ��һ����NaCl��������Na2CO3�����ᣮ[ʵ��̽��]
��һ��ȷ����Һ���Ƿ������
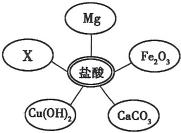
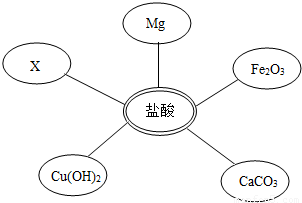
��1��ѡ���Լ�����������Ļ�ѧ���ʣ�ͬѧ��ѡ������ͼ��ʾ���������ʣ���������x�����ָʾ���е�
ʯ��
ʯ��
��Һ����2��ʵ����֤��ijͬѧ���Һ�м���������þ�ۣ��۲쵽ȷ����Һ��һ��û�����ᣮ
������ȷ����Һ���Ƿ���Na2CO3ijͬѧѡ��
pH��ֽ����pH�ƣ�
pH��ֽ����pH�ƣ�
�����Һ��pH=l0��ȷ����Һ��һ������Na2CO3��������������Һ�������������ӷ�Һ�еõ�������NaCl�����������ʵ�鷽����ƣ�
| ���� | �����Լ� | ���뷽�� | �������� |
| ����Ca��NO3��2��Һ | ���ˡ������ᾧ | �����У������� ���������ʣ�����NaNO3�����ɻ�����NO�����ӣ� ���������ʣ�����NaNO3�����ɻ�����NO�����ӣ� | |
| ������ ���ᣨ��ϡHCl��HCl�� ���ᣨ��ϡHCl��HCl�� | �����ᾧ | ���� |
������[��������]����̼���������ᷴӦ��ʵ��д���䷴Ӧ�Ļ�ѧ����ʽ��
[ʵ��̽��]
��һ����1���ж���Һ���Ƿ�������ȡ�õ����ָʾ�������÷�̪�Լ��������ʹ��ɫ�ķ�̪��ɫ����2����ȷþ�����ᷴӦ���ֵ�����
�������ⶨ��Һ������Ե��������pH��ֽ��
���������ݻ�ѧ�ϳ�����ԭ�����ʵ�鷽����ƣ�����ȥԭ���ʲ��������µ����ʣ�
[ʵ��̽��]
��һ����1���ж���Һ���Ƿ�������ȡ�õ����ָʾ�������÷�̪�Լ��������ʹ��ɫ�ķ�̪��ɫ����2����ȷþ�����ᷴӦ���ֵ�����
�������ⶨ��Һ������Ե��������pH��ֽ��
���������ݻ�ѧ�ϳ�����ԭ�����ʵ�鷽����ƣ�����ȥԭ���ʲ��������µ����ʣ�
����⣺[��������]����̼���������ᷴӦ��ʵ��д���䷴Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl=2NaCl+H2O+CO2����
[ʵ��̽��]��һ��
��1���ж���Һ���Ƿ�������ȡ�õ����ָʾ�������÷�̪�Լ��������ʹ��ɫ�ķ�̪��ɫ����ȡ��ʯ����Һ������ʹʯ����Һ���ɫ��
��2��þ�����ᷴӦ���ֵ������Dz����������ݣ���û���ݲ�����֤����Һ��һ��û�����
�������ⶨ��Һ������Ե��������pH��ֽ��Ҳ��ʹ��pH�ƣ���
���������ݻ�ѧ�ϳ�����ԭ�����ʵ�鷽����ƣ�����ȥԭ���ʲ��������µ����ʣ����������Һ���������÷�Һ�еõ�������NaClʱ���������Ca��NO3��2��Һ����Ȼ��ȥ��̼���ƣ���ȴ����������NaNO3������������ϡ���������ᾧ��ɵõ��������Ȼ��ƣ�
�ʴ�Ϊ��
[��������]Na2CO3+2HCl=2NaCl+H2O+CO2��
[ʵ��̽��]
��һ��
��1��ʯ��
��2�������ݲ���������������ޱ仯��
������pH��ֽ����pH�ƣ�
[ʵ��̽��]��һ��
��1���ж���Һ���Ƿ�������ȡ�õ����ָʾ�������÷�̪�Լ��������ʹ��ɫ�ķ�̪��ɫ����ȡ��ʯ����Һ������ʹʯ����Һ���ɫ��
��2��þ�����ᷴӦ���ֵ������Dz����������ݣ���û���ݲ�����֤����Һ��һ��û�����
�������ⶨ��Һ������Ե��������pH��ֽ��Ҳ��ʹ��pH�ƣ���
���������ݻ�ѧ�ϳ�����ԭ�����ʵ�鷽����ƣ�����ȥԭ���ʲ��������µ����ʣ����������Һ���������÷�Һ�еõ�������NaClʱ���������Ca��NO3��2��Һ����Ȼ��ȥ��̼���ƣ���ȴ����������NaNO3������������ϡ���������ᾧ��ɵõ��������Ȼ��ƣ�
�ʴ�Ϊ��
[��������]Na2CO3+2HCl=2NaCl+H2O+CO2��
[ʵ��̽��]
��һ��
��1��ʯ��
��2�������ݲ���������������ޱ仯��
������pH��ֽ����pH�ƣ�
| ���� | �����Լ� | ���뷽�� | �������� |
| һ | ���������ʣ�����NaNO�����ɻ�����NOi�����ӣ� | ||
| �� | ���ᣨ��ϡHCl��HCl�� |
���������⿼��̽��������ʼ���д�йػ�ѧ����ʽ��������ƿ�ѧ�������ܺܺõ�����ѧ�������֪ʶϵͳ�������ٻ�е���䣬��������ļ�������ε�֪ʶ�㣮

��ϰ��ϵ�д�
 �¿α�����Ķ�ѵ��ϵ�д�
�¿α�����Ķ�ѵ��ϵ�д�
�����Ŀ
��2011?���죩ͬѧ��Ϊ̽����ͬ�����Ļ��ǿ�������������ʵ�鷽�������з�����ʵ����ơ������۾���ȷ��һ���ǣ�������
|
��2011?���֣�ͬѧ����Na2CO3��Һ��ŨHCl���о�����������ķ�Ӧԭ��ʱ���Է�Һ�ijɷֽ���̽����
[��������]
�����������ʷ�����Ӧ�Ļ�ѧ����ʽΪ______���ɴ��Ʋ����Һ��һ����NaCl��������Na2CO3�����ᣮ
[ʵ��̽��]
��һ��ȷ����Һ���Ƿ������
��1��ѡ���Լ�����������Ļ�ѧ���ʣ�ͬѧ��ѡ������ͼ��ʾ���������ʣ���������x�����ָʾ���е�______��Һ��
��2��ʵ����֤��ijͬѧ���Һ�м���������þ�ۣ��۲쵽______ȷ����Һ��һ��û�����ᣮ
������ȷ����Һ���Ƿ���Na2CO3ijͬѧѡ��______�����Һ��pH=l0��ȷ����Һ��һ������Na2CO3��
������������Һ�������������ӷ�Һ�еõ�������NaCl�����������ʵ�鷽����ƣ�
[��������]
�����������ʷ�����Ӧ�Ļ�ѧ����ʽΪ______���ɴ��Ʋ����Һ��һ����NaCl��������Na2CO3�����ᣮ
[ʵ��̽��]
��һ��ȷ����Һ���Ƿ������
��1��ѡ���Լ�����������Ļ�ѧ���ʣ�ͬѧ��ѡ������ͼ��ʾ���������ʣ���������x�����ָʾ���е�______��Һ��
��2��ʵ����֤��ijͬѧ���Һ�м���������þ�ۣ��۲쵽______ȷ����Һ��һ��û�����ᣮ
������ȷ����Һ���Ƿ���Na2CO3ijͬѧѡ��______�����Һ��pH=l0��ȷ����Һ��һ������Na2CO3��
������������Һ�������������ӷ�Һ�еõ�������NaCl�����������ʵ�鷽����ƣ�
| ���� | �����Լ� | ���뷽�� | �������� |
| һ | ����Ca��NO3��2��Һ | ���ˡ������ᾧ | �����У������� ______ |
| �� | ������______ | �����ᾧ | ���� |

 ��2011?���죩ͬѧ���������ͼ��ʾװ����̽��������̼����ȡ�����ʣ����й��ڸ�ʵ���������ȷ���ǣ�������
��2011?���죩ͬѧ���������ͼ��ʾװ����̽��������̼����ȡ�����ʣ����й��ڸ�ʵ���������ȷ���ǣ�������