��Ŀ����
 ��ѧ�����������������ż������еĹ�ϵ�����ѧ���ʶԴٽ����彡�������Ƽ�������Ҫ�����ã�������ijҩ��˵����IJ������֣�����ϸ�Ķ���ش�
��ѧ�����������������ż������еĹ�ϵ�����ѧ���ʶԴٽ����彡�������Ƽ�������Ҫ�����ã�������ijҩ��˵����IJ������֣�����ϸ�Ķ���ش���1����̪����
��2����̪�����и�Ԫ�ص�ԭ�Ӹ�����Ϊ
��3�������������������øù���Ƭ��һ������ķ�̪����Ϊ
��4����ʽ���㣺
�ٷ�̪����Է���������
�ڷ�̪��̼Ԫ�ص�����������������0.1%����
���㣺��ǩ�ϱ�ʾ�����ʳɷּ��京��,��ѧʽ����д������,��Է��������ĸ�������,Ԫ�������ȵļ���,Ԫ�ص�������������
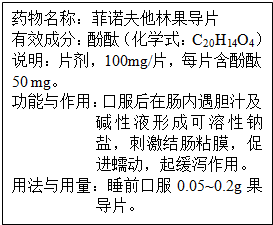
ר�⣺��ǩͼʾ��
��������1���ӷ�̪�Ļ�ѧʽΪ��C20H14O4������C��H��O����Ԫ����ɵĴ�������ڻ�������ݻ�ѧʽ�����и�Ԫ�ص�����֮��ȥ�������
��2���ӻ�ѧʽ��Ԫ�ط������½ǵ����ִ��������ڵ�ԭ�Ӹ���ȥ�������
��3����˵������Է��֣�ÿƬ��ŵ�����ֹ���Ƭ��100mg������50mg��̪ȥ�������
��4�����ݷ�̪�Ļ�ѧʽ������صļ��㣮
��2���ӻ�ѧʽ��Ԫ�ط������½ǵ����ִ��������ڵ�ԭ�Ӹ���ȥ�������
��3����˵������Է��֣�ÿƬ��ŵ�����ֹ���Ƭ��100mg������50mg��̪ȥ�������
��4�����ݷ�̪�Ļ�ѧʽ������صļ��㣮
����⣺��1����̪�Ļ�ѧʽΪ��C20H14O4������C��H��O����Ԫ����ɵĴ�������ڻ�������и�Ԫ�ص�����֮��Ϊ��C��H��O=��12��20������1��14������16��4��=120��7��32���ʴ�Ϊ��3 ������ C��H��O=120��7��32��
��2����ѧʽ��Ԫ�ط������½ǵ����ִ��������ڵ�ԭ�Ӹ������ɷ�̪�Ļ�ѧʽ���Կ�����һ����̪�����к���20��̼ԭ�ӡ�14����ԭ�Ӻ�4����ԭ�ӣ����Ը�Ԫ�ص�ԭ�Ӹ����ȣ�C��H��O=20��14��4=10��7��2���ʴ�Ϊ��C��H��O=10��7��2��
��3����˵������Է��֣�ÿƬ��ŵ�����ֹ���Ƭ��100mg������50mg��̪���������������������øù���Ƭ����0.2g��200mg������Ƭ�����з�̪������Ϊ��
��50mg=100mg=0.1g���ʴ�Ϊ��0.1g��
��4���ٷ�̪����Է�������=12��20+1��14+16��4=318��
�ڷ�̪��̼Ԫ�ص���������=
��100%=
��100%=75.5%��
�ʴ�Ϊ��318 75.5%��
��2����ѧʽ��Ԫ�ط������½ǵ����ִ��������ڵ�ԭ�Ӹ������ɷ�̪�Ļ�ѧʽ���Կ�����һ����̪�����к���20��̼ԭ�ӡ�14����ԭ�Ӻ�4����ԭ�ӣ����Ը�Ԫ�ص�ԭ�Ӹ����ȣ�C��H��O=20��14��4=10��7��2���ʴ�Ϊ��C��H��O=10��7��2��
��3����˵������Է��֣�ÿƬ��ŵ�����ֹ���Ƭ��100mg������50mg��̪���������������������øù���Ƭ����0.2g��200mg������Ƭ�����з�̪������Ϊ��
| 200mg |
| 100mg |
��4���ٷ�̪����Է�������=12��20+1��14+16��4=318��
�ڷ�̪��̼Ԫ�ص���������=
| ̼Ԫ�ص����ԭ��������ԭ�Ӹ��� |
| ��̪����Է������� |
| 12��20 |
| 318 |
�ʴ�Ϊ��318 75.5%��
������������Ҫ�������йػ�ѧʽ��ʾ�����弰���ݻ�ѧʽ���м��㣬��ѧ�û�ѧ�Ļ�����

��ϰ��ϵ�д�
�����Ŀ

 ��ʾ����
��ʾ����