��Ŀ����
30��ʱ��NaOH�ܽ���ˮ��ʵ���������±���
| ʵ����� | ˮ���������ˣ� | ����NaOH�������ˣ� | ������Һ�������ˣ� |
| �� | 10 | 5 | 15 |
| �� | 10 | 10 | 20 |
| �� | 10 | 15 | 21 |
| �� | 10 | 20 | 21 |
��2��ʵ������õ���Һ��______��Һ����ͻ��ͣ���
��3��30��ʱ��NaOH���ܽ��Ϊ______��/100��ˮ
��4�������ʵ����У��μ�����������ͭ��Һ���������______mol�������÷�Ӧ�Ļ�ѧ����ʽ______��
�⣺
��1��ʵ����У�5gNaOH��ȫ������10gˮ�γ�15g��Һ������Һ����������Ϊ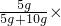 100%=33.3%��
100%=33.3%��
��2���ԱȢڢ۵�ʵ�����ݣ�����15g������ʱ������ҺΪ21g������ʵ��ڵ�20g��Һ��˵��10gˮ�ܽ�10g������ʱ��δ�ﵽ����״̬���ʢ���������Һ�Dz�������Һ��
��3������ʵ����������ݣ�30��ʱ10gˮ�ܽ�21g-10g=11g������ʱ��ҺΪ������Һ����30��ʱ10gˮ�к���11g�����ʣ���30��ʱ��NaOH���ܽ��Ϊ110g��
��4��������ij���������Ϊx��
2NaOH+CuSO4�TNa2SO4+Cu��OH��2��
80 98
10g x
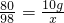 x=
x=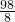 g
g
 =0.125mol��
=0.125mol��
�ʴ�Ϊ����1��33.3%����2�������ͣ���3��110����4��0.125��2NaOH+CuSO4�TNa2SO4+Cu��OH��2����
�������������ݱ����Ĵ�ʵ���ܼ������������ʼ���Һ�����ı仯���������ʵ��ܽ���������ݶ������ܽ�����ķ����жϽ��
���������⡰������Һ��ָ��һ���¶��¡�һ�������ܼ�������ټ����ܽ��������ʵ���Һ����Һ���͵ı�־����Һ���й���ʣ�࣬��ʣ�����������ټ��١��ǽ������Ĺؼ���
��1��ʵ����У�5gNaOH��ȫ������10gˮ�γ�15g��Һ������Һ����������Ϊ
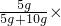 100%=33.3%��
100%=33.3%����2���ԱȢڢ۵�ʵ�����ݣ�����15g������ʱ������ҺΪ21g������ʵ��ڵ�20g��Һ��˵��10gˮ�ܽ�10g������ʱ��δ�ﵽ����״̬���ʢ���������Һ�Dz�������Һ��
��3������ʵ����������ݣ�30��ʱ10gˮ�ܽ�21g-10g=11g������ʱ��ҺΪ������Һ����30��ʱ10gˮ�к���11g�����ʣ���30��ʱ��NaOH���ܽ��Ϊ110g��
��4��������ij���������Ϊx��
2NaOH+CuSO4�TNa2SO4+Cu��OH��2��
80 98
10g x
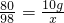 x=
x=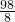 g
g  =0.125mol��
=0.125mol���ʴ�Ϊ����1��33.3%����2�������ͣ���3��110����4��0.125��2NaOH+CuSO4�TNa2SO4+Cu��OH��2����
�������������ݱ����Ĵ�ʵ���ܼ������������ʼ���Һ�����ı仯���������ʵ��ܽ���������ݶ������ܽ�����ķ����жϽ��
���������⡰������Һ��ָ��һ���¶��¡�һ�������ܼ�������ټ����ܽ��������ʵ���Һ����Һ���͵ı�־����Һ���й���ʣ�࣬��ʣ�����������ټ��١��ǽ������Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
���и����У�ǰ��һ�����ں��ߵ��ǣ�������
| A��NaOH����ˮ����Һ���¶ȡ�NH4NO3����ˮ����Һ���¶� | B��30��Cʱ��NaCl��������Һ������������NaCl������Һ���������� | C��10%��ϡ�����pH��10%������������Һ��pH | D��10gþ���������ᷴӦ����H2��������10gп���������ᷴӦ����H2������ |


