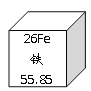
��Ŀ����
����Ŀ��Ԫ�����ڱ��У�ͬ����Ԫ�صĽṹ�����ʳ���һ���Ĺ����Ա仯���±��г����ǵ�������Ԫ�ص���Ҫ���ϼۣ�������Ϣδ�г���������Ӧ�Ļ�ѧ������ա�
Ԫ�� | Na | Mg | �� | Si | P | �� | Cl |
ԭ�ӵ����������� | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
������� ����� | ��1 | ��2 | �� | ��4 ��4 | ��5 ��3 | �� | ��7 ��1 |
��1����Ԫ�ص�������_____________�ٺ͢�Ԫ���γɵĻ�����Ļ�ѧʽΪ__________
��2���۴���Ļ��ϼ�Ϊ ______________
��3���ܴ�����������ǣ�6��������� ______���ɴ�����Եó��й�Ԫ�ػ��ϼ۵�һ������Ϊ________________��
Ԫ��X��Y��Z��M�dz��г���������Ԫ�ء��й���Ϣ���±���
Ԫ�� | �й���Ϣ |
X | ���γ���Է���������С�����嵥�� |
Y | �����к�������Ԫ�� |
Z | �䵥��Լռ�����������1\5 |
M | ������������������Ľ���Ԫ�� |
����X��Y����Ԫ����ɵ����������_________________���ѧʽ��
����X��Z����Ԫ�ذ�ԭ�Ӹ�����1��1��ɵĻ����д�������ٷֽⷴӦ�Ļ�ѧ����ʽ______________________��
��д��M�ĵ�����������ȼ�յĻ�ѧ����ʽ________________________��
���𰸡� �� Al2S3 +3 -2 �������������۵ľ���ֵ֮�͵���8���������ɣ� NH3 2H2O2![]() 2H2O+O2�� 3Fe+2O2
2H2O+O2�� 3Fe+2O2 ![]() Fe3O4
Fe3O4
��������������ѧ֪ʶ��������Ϣ֪����1����Ԫ�ص����������ٺ͢�Ԫ���γɵĻ�����Ļ�ѧʽΪ Al2S3�� ��2���۴���Ļ��ϼ�Ϊ+3����3���ܴ�����������ǣ�6���������-2���й�Ԫ�ػ��ϼ۵�һ������Ϊ�������������۵ľ���ֵ֮�͵���8����Ԫ��X��Y��Z��M�dz��г���������Ԫ�ء�����X��Y����Ԫ����ɵ����������NH3�� ����X��Z����Ԫ�ذ�ԭ�Ӹ�����1��1��ɵĻ���������ٷֽⷴӦ�Ļ�ѧ����ʽ��2H2O2 ![]() 2H2O+O2������M�ĵ�����������ȼ�յĻ�ѧ����ʽ�� 3Fe+2O2��ȼFe3O4��
2H2O+O2������M�ĵ�����������ȼ�յĻ�ѧ����ʽ�� 3Fe+2O2��ȼFe3O4��

 ����ν����Ž̲��㽭���̴�ѧ������ϵ�д�
����ν����Ž̲��㽭���̴�ѧ������ϵ�д� �����Ļ������������������ϵ�д�
�����Ļ������������������ϵ�д�