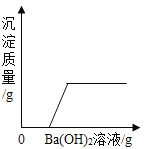
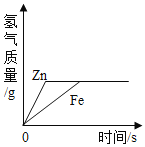
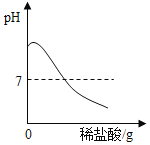
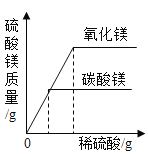
��Ŀ����
����Ŀ������ʹ�û��ʿ����ũ�����������ͼ���������ص���ʾ��ͼ���ش����⡣
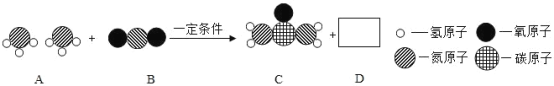
��1��A ��������Ԫ�صĻ��ϼ���_________��D ���ʵĻ�ѧʽ��_________��
��2�����������ز�����ͬ����ϵ���________��
ANH4HCO3 BNH4Cl CK2SO4
��3������ʩ�û��ʿ�������ʳ���������õĻ�����K2SO4��CO��NH2��2��Ca��H2PO4��2��KNO3�ȣ��������ڸ��Ϸʵ���_________��
��4��ũ��������������������һ����Ҫ����Щ����������Ҫ������ͨ��������ʩ��_________������ţ����� �ܸ�����������������ǿ��������
A��ʯ�� B�����[Ca3��H2PO4��2] C��ľ�ң���Ҫ�ɷ�̼��� K2CO3��
���𰸡�+1 H2O C KNO3 C
��������
��1�����ݹ�ҵ���������صķ�Ӧ����ʾ��������غ㶨�ɿ�֪��A��B��C��D�ֱ�ΪNH3��CO2��CO��NH2��2��H2O����Ӧ�ķ���ʽ�ǣ�2NH3+CO2 CO��NH2��2+H2O���ɴ˿�֪��A������NH3��NH3����Ԫ�صĻ��ϼ���+1��D���ʵĻ�ѧʽ��H2O��
CO��NH2��2+H2O���ɴ˿�֪��A������NH3��NH3����Ԫ�صĻ��ϼ���+1��D���ʵĻ�ѧʽ��H2O��
��2������[CO(NH2)2] �к��е�Ԫ�����ڵ��ʡ�
A��NH4HCO3�к��е�Ԫ�����ڵ��ʣ�
B��NH4Cl�к��е�Ԫ�����ڵ��ʣ�
C��K2SO4�к��м�Ԫ�����ڼطʣ������ز�����ͬ����ϵ���K2SO4����ѡC��
��3�����е�Ԫ�صķ��ϳ�Ϊ���ʡ�������Ԫ�صķ��ϳ�Ϊ�ʡ����м�Ԫ�صķ��ϳ�Ϊ�طʣ�ͬʱ���е����ס�������Ԫ���е����ֻ��������ϵķ��ϳ�Ϊ���Ϸʣ���K2SO4���ڼطʡ�[CO(NH2)2]���ڵ��ʡ�Ca��H2PO4��2�����ʣ�KNO3��ͬʱ���м�Ԫ�غ͵�Ԫ�أ����ڸ��Ϸʣ�
��4�����������������õ�������������Σ��ط��ܹ����������������������Dz�ľ�ҡ���ѡC��

 ����ʦ��Сһ����ʦ������ҵϵ�д�
����ʦ��Сһ����ʦ������ҵϵ�д�����Ŀ�����г���(������Ϊ����)���Լ�ѡ����ȷ����( )
��� | ���� | ѡ���Լ� |
A | CaCl2��Һ(����) | ������Na2CO3��Һ |
B | NaOH��Һ(Na2CO3��Һ) | ������Ca(OH)2��Һ |
C | CuO(Cu) | ������ϡH2SO4 |
D | KCl��Һ(K2SO4��Һ) | ������Ba(NO3)2��Һ |
A.AB.BC.CD.D