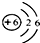��Ŀ����
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�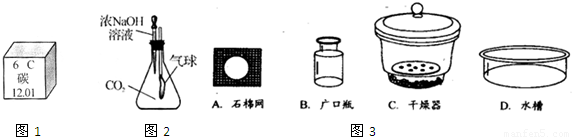
��1������̼��������������������ģ���Ҫ��Ϊ�˼���______���ѧʽ�����ŷ�����
��2����ͼ1ΪԪ�����ڱ��е�һ������˵������ȷ����______�����ţ���
A��̼Ԫ�����ڷǽ���Ԫ��
B��̼ԭ�Ӻ���������Ϊ6
C��̼Ԫ�ص�ԭ�ӽṹʾ��ͼΪ
D��̼�����ԭ������Ϊ12.01
��3����ͼ2��ijȤζʵ��װ��ͼ����ѹ��ͷ�ιܺɹ۲쵽�����ʹ�������������ԭ��д����ѧ����ʽ��
��4����֪̼��������270�����Ҿ��ֽܷ�Ϊ̼���ơ�ˮ�Ͷ�����̼����̼�������Ȳ��ֽ⣮����ij������������һ��̼�������л���������̼���ƣ�Ϊ�˲ⶨ��Ʒ��̼��������������������ļ��鲽�����£�
��ȡһֻ�ྻ����������������Ϊag���������м�����Ʒ���Ƶ�������Ϊm1g��
�ڼ��ȸ�ʢ����Ʒ��������
�۽����������ȴ������������ʣ������������
�ܶ���ظ�����ں͢������أ��Ƶ�������ʣ������������Ϊm2g��
��1��д��̼���������ȷֽ�Ļ�ѧ����ʽ______ Na2CO3+H2O��+CO2��
���𰸡���������1�����ݡ���̼��������������������ģ����ٺ�̼���ʵ��ͷţ���Ҫ��ָ����CO2���ŷ������н��
��2������Ԫ�����ڱ������ṩ����Ϣ���н��
��3����������������Һ�Ͷ�����̼��Ӧ����̼���ƺ�ˮʹƿ��ѹǿ��С���н��
��4������1��������Ϣ��̼��������270�����Ҿ��ֽܷ�Ϊ̼���ơ�ˮ�Ͷ�����̼��д��̼���������ȷֽ�Ļ�ѧ����ʽ��
��2��Ϊ��ֹ���Ⱥ���������ȴ���������տ�����ˮ��Ӱ��ⶨ�����Ӧ���ڸ������н�����ȴ��
��3�����������غ㶨�ɣ��ɼ���ǰ��ʣ�����ʵ����������÷ֽⷴӦ��������̼��ˮ��������������ٵ��������ɹ�����ٵ��������ݻ�ѧ����ʽ�������Ʒ��̼����������������һ��������Ʒ��̼�����Ƶ�����������
��4�����ݼ�����Ʒ��̼�����Ƶ���������Ϊ90%��������ٵ�����0.6g������Ʒ��̼�����Ƶ����������Ĵ���ʽ����������������Ʒ��������
����⣺��1������̼��������������������ģ����ٺ�̼���ʵ��ͷţ���Ҫ��ָ����CO2���ŷ�����
��2��A��̼Ԫ�����ڷǽ���Ԫ�أ���A��ȷ��
B��̼ԭ�Ӻ���������Ϊ6����B��ȷ
C��̼ԭ�Ӻ���������Ϊ6��֪̼Ԫ�ص�ԭ�ӽṹʾ��ͼΪ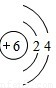 ����C����
����C����
D����ͼ1��֪̼�����ԭ������Ϊ12.01����D��ȷ��
��3������������Һ�Ͷ�����̼��Ӧ����̼���ƺ�ˮ��ʹƿ��ѹǿ��С�����Կɹ۲쵽�����ʹ�ѧ����ʽ2NaOH+CO2=Na2CO3+H2O��
��4������1��̼��������270�����Ҿ��ֽܷ�Ϊ̼���ơ�ˮ�Ͷ�����̼���ڸ��¶���ˮ����̬�ģ����Է�Ӧ�Ļ�ѧ����ʽΪ2NaHCO3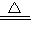 Na2CO3+H2O��+CO2����
Na2CO3+H2O��+CO2����
��2��ʹ������ǯ�Ѽ��Ⱥ���������������C�н�����ȴ����ֹ��ȴ���������տ����е�ˮ�֣���Ӱ�����ij����Ľ����
��3������Ʒ��̼�����Ƶ�����Ϊx
2NaHCO3 Na2CO3+H2O��+CO2�� ������������
Na2CO3+H2O��+CO2�� ������������
168 106 168-106=62
x m1-m2
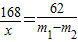
x=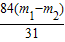
��Ʒ��̼�����Ƶ���������=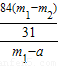 ×100%=
×100%=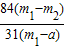 ×100%��
×100%��
��4��������Ʒ��̼�����Ƶ���������Ϊ90%��������ٵ�����0.6g������Ʒ��̼�����Ƶ����������Ĵ���ʽ����֪��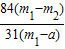 ×100%=90%
×100%=90%
��m1-a��=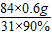 =1.8g
=1.8g
���������Ʒ������Ϊ1.8g��
�ʴ�Ϊ����1��CO2����2��C����3������������Һ�Ͷ�����̼��Ӧ����̼���ƺ�ˮ��ʹƿ��ѹǿ��С��2NaOH+CO2=Na2CO3+H2O����4������1��2NaHCO3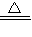 Na2CO3+H2O��+CO2������2������ǯ��C����3��
Na2CO3+H2O��+CO2������2������ǯ��C����3��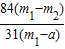 ×100%����4��1.8g��
×100%����4��1.8g��
���������ʱ��Ҫע��������ڼ���ʱ������С�����ڷֽ����ɶ�����̼��ˮ��ԭ����ˣ�����ʱ���ܰѴ����������ų�������̼���������㣮
��2������Ԫ�����ڱ������ṩ����Ϣ���н��
��3����������������Һ�Ͷ�����̼��Ӧ����̼���ƺ�ˮʹƿ��ѹǿ��С���н��
��4������1��������Ϣ��̼��������270�����Ҿ��ֽܷ�Ϊ̼���ơ�ˮ�Ͷ�����̼��д��̼���������ȷֽ�Ļ�ѧ����ʽ��
��2��Ϊ��ֹ���Ⱥ���������ȴ���������տ�����ˮ��Ӱ��ⶨ�����Ӧ���ڸ������н�����ȴ��
��3�����������غ㶨�ɣ��ɼ���ǰ��ʣ�����ʵ����������÷ֽⷴӦ��������̼��ˮ��������������ٵ��������ɹ�����ٵ��������ݻ�ѧ����ʽ�������Ʒ��̼����������������һ��������Ʒ��̼�����Ƶ�����������
��4�����ݼ�����Ʒ��̼�����Ƶ���������Ϊ90%��������ٵ�����0.6g������Ʒ��̼�����Ƶ����������Ĵ���ʽ����������������Ʒ��������
����⣺��1������̼��������������������ģ����ٺ�̼���ʵ��ͷţ���Ҫ��ָ����CO2���ŷ�����
��2��A��̼Ԫ�����ڷǽ���Ԫ�أ���A��ȷ��
B��̼ԭ�Ӻ���������Ϊ6����B��ȷ
C��̼ԭ�Ӻ���������Ϊ6��֪̼Ԫ�ص�ԭ�ӽṹʾ��ͼΪ
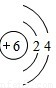 ����C����
����C����D����ͼ1��֪̼�����ԭ������Ϊ12.01����D��ȷ��
��3������������Һ�Ͷ�����̼��Ӧ����̼���ƺ�ˮ��ʹƿ��ѹǿ��С�����Կɹ۲쵽�����ʹ�ѧ����ʽ2NaOH+CO2=Na2CO3+H2O��
��4������1��̼��������270�����Ҿ��ֽܷ�Ϊ̼���ơ�ˮ�Ͷ�����̼���ڸ��¶���ˮ����̬�ģ����Է�Ӧ�Ļ�ѧ����ʽΪ2NaHCO3
 Na2CO3+H2O��+CO2����
Na2CO3+H2O��+CO2������2��ʹ������ǯ�Ѽ��Ⱥ���������������C�н�����ȴ����ֹ��ȴ���������տ����е�ˮ�֣���Ӱ�����ij����Ľ����
��3������Ʒ��̼�����Ƶ�����Ϊx
2NaHCO3
 Na2CO3+H2O��+CO2�� ������������
Na2CO3+H2O��+CO2�� ������������168 106 168-106=62
x m1-m2
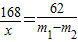
x=
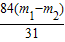
��Ʒ��̼�����Ƶ���������=
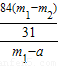 ×100%=
×100%=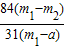 ×100%��
×100%����4��������Ʒ��̼�����Ƶ���������Ϊ90%��������ٵ�����0.6g������Ʒ��̼�����Ƶ����������Ĵ���ʽ����֪��
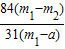 ×100%=90%
×100%=90%��m1-a��=
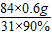 =1.8g
=1.8g���������Ʒ������Ϊ1.8g��
�ʴ�Ϊ����1��CO2����2��C����3������������Һ�Ͷ�����̼��Ӧ����̼���ƺ�ˮ��ʹƿ��ѹǿ��С��2NaOH+CO2=Na2CO3+H2O����4������1��2NaHCO3
 Na2CO3+H2O��+CO2������2������ǯ��C����3��
Na2CO3+H2O��+CO2������2������ǯ��C����3��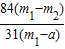 ×100%����4��1.8g��
×100%����4��1.8g�����������ʱ��Ҫע��������ڼ���ʱ������С�����ڷֽ����ɶ�����̼��ˮ��ԭ����ˣ�����ʱ���ܰѴ����������ų�������̼���������㣮

��ϰ��ϵ�д�
 ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д�
�����Ŀ
 ̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ� ̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
 ̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ� D��̼�����ԭ������Ϊ12.01
D��̼�����ԭ������Ϊ12.01
 ̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�
̼���γɻ�������������Ԫ�أ���̼��������ѧ��ѧ�о�����Ҫ���ݣ�