��Ŀ����
��ѧ��ȤС���ͬѧ��ʵ���Ҳⶨij������ʯ��������������������ȡ������ʯ��Ʒ10.0 g������ϡ����109.5 g��ǡ����ȫ��Ӧ�����˵õ�����2.0 g���������ʼȲ�����ˮҲ�����ᷢ����Ӧ��������㣺
��1��������ʯ��������������������
��2����Ӧ��������Һ�����ʵ�����������
����Ӧ�Ļ�ѧ����ʽΪ��Fe2O3 + 6HCl =2 FeCl3 + 3H2O����������ȷ��0.1%��д����Ҫ�ļ�����̡������õ������ԭ��������H—1��C—12��O—16��Cl—35.5�� Fe—56��
�⣨1��������ʯ������������������ = (10.0g-2.0g)/10.0g ��100% = 80.0%��1�֣�
��2���跴Ӧ����Һ�����ʵ�����Ϊx
Fe2O3 + 6HCl =2 FeCl3 + 3H2O 
160 2��162.5
8.0g x ��1�֣�
160��8.0g��2��162.5��x
x �� 16.25 g ��2�֣�
��Һ�����ʵ�����������16.25 g����109��5 g + 8.0g����100% ��13.8 % ��2�֣�
����

 20��ijУ��ѧ��ȤС���ͬѧ���Ķ�����ʱ���֣�18����ĩ��������ѧ��������������������-��ʵ�飺��ˮ����ͨ��һ���պ��ǹ�ܣ�������һ�����壮ͬѧ�Ƕ����ʵ��ܸ���Ȥ���ѵ����ȵ�������ˮ������Ӧ�����ɵ���������ʲô��������Щ���ʣ��������������ͼ��ʾ��װ�ý���̽����
20��ijУ��ѧ��ȤС���ͬѧ���Ķ�����ʱ���֣�18����ĩ��������ѧ��������������������-��ʵ�飺��ˮ����ͨ��һ���պ��ǹ�ܣ�������һ�����壮ͬѧ�Ƕ����ʵ��ܸ���Ȥ���ѵ����ȵ�������ˮ������Ӧ�����ɵ���������ʲô��������Щ���ʣ��������������ͼ��ʾ��װ�ý���̽����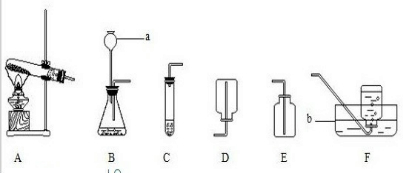
 ��2012?̫ԭ��ij��ѧ��ȤС���ͬѧ��̽��Mg��Cu��Fe���ֽ������й�����ʱ����������ʵ�飺
��2012?̫ԭ��ij��ѧ��ȤС���ͬѧ��̽��Mg��Cu��Fe���ֽ������й�����ʱ����������ʵ�飺