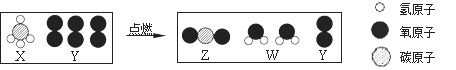
��Ŀ����
����Ŀ��(1)����������������и�С��:̼��ơ������ơ��������ơ�̼�����ơ�̼���ơ��Ȼ���(�û�ѧʽ��д)��
�����г�����ʳƷ���������_______�������������Ƽ�����______��
�������ܺ����ᷢ���кͷ�Ӧ����____������ũҵ�ϵ�һ����Ҫ��;��_______��
�����п����ڱ��Ƹ�����______������ָ����_______��
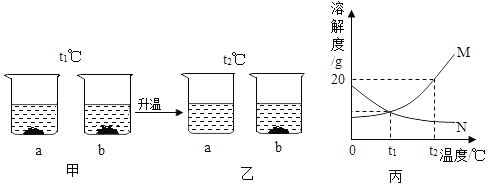
(2)t1��Cʱ,��a��b���ֹ����18g,�ֱ���˵�ʢ��100gˮ���ձ���,��ֽ����������ͼ����ʾ,���µ�t2��Cʱ,������ͼ����ʾ,a��b���ֹ�����ˮ�е��ܽ��������ͼ����ʾ��
�����:
�� ��t1��C��t2�� C�ı仯������,һֱ���ڱ���״̬����___(����a������b��)����Һ��
��ͼ��������M��ʾ����_________(����a������b��)���ܽ�����ߡ�
����t2��Cʱ200gˮ�м���50g��a����ܽ��������Һ����________g.������Һ����������������________(����һλС��)��
���𰸡�CaO CaCO3 Ca(OH)2 ������������ NaHCO3 Na2CO3 b a 240 16.7%
��������
(1) ������������ˮ��Ӧ��������ʳƷ���������CaO�������������Ƽ����ǣ�CaCO3��
�������ܺ����ᷢ���ᡢ���кͷ�Ӧ���ǣ�Ca(OH)2������ũҵ�ϵ�һ����Ҫ��;�ǣ���������������
�����п����ڱ��Ƹ�����С�մ�NaHCO3������ָ����:Na2CO3��
(2) �ٴ�t1��C��t2�� C�ı仯������,b�ձ���ʼ���й���δ�ܽ⣬һֱ���ڱ���״̬����b����Һ��
��ͼ��������M��ʾ�����¶ȵ����ߣ��ܽ�ȱ��M��ʾa���ʵ��ܽ�����ߡ�
����t2��Cʱ��a���ʵ��ܽ��Ϊ20g����ÿ100gˮ�������ܽ�20g a���ʣ���ô200gˮ�м���50g��ֻ���ܽ�40g��a����ܽ��������Һ����=200g+40g=240g��
������Һ����������������=![]() ��
��