��Ŀ����
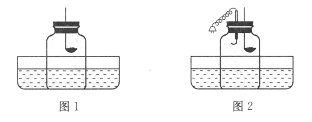
����Ŀ����5�֣���ʵ��ɽ�ԼҩƷ����ʡʱ�䣬������ȫ����Ⱦ�١���ͼ�� Y �ιܻ� Y �ε����������ʵ�顣
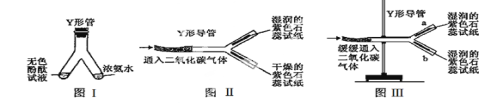
��1��ͼ���� Y �ι������м�����ɫ��̪��Һ���Ҳ���м���Ũ��ˮ��һ��ʱ��ɹ۲쵽������ ���÷��ӵĹ۵������һ����_______
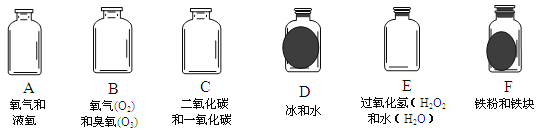
��2��ͼ���� Y �ε���ƽ�������棬ʵ���пɹ۲쵽������______________________���÷��ű���ʽ���ͽ��ۣ� ��
��3������Y�ε��̶ܹ�������̨�ϣ���ͼ �� ) , a ��λ���Ϸ���b ��λ���·�������ʯ����ֽ��ʪ�ɹ۲쵽b������ֽ��ɫ��a �����ԣ�ԭ�� ��
���𰸡���1����ɫ�ķ�̪��� �����ڲ�ͣ���˶�
��2��ʪ�����ɫʯ����ֽ���ɫ���������ɫʯ����ֽ�����Ա仯H2O+CO2![]() H2 CO3
H2 CO3
��3��������̼���ܶȱȿ�����
��������
�����������1��Ũ��ˮ��ʹ��ɫ��̪��죬ͼ���� Y �ι������м�����ɫ��̪��Һ���Ҳ���м���Ũ��ˮ��һ��ʱ��ɹ۲쵽��������ɫ�ķ�̪��죬ԭ���ǣ��������ڲ�ͣ���˶����˶�����ɫ��̪��Һ�У�ʹ����
��2�����ڶ�����̼����ˮ��Ӧ��̼�ᣬ��ͼ���� Y �ε���ƽ�������棬ʵ���пɹ۲쵽������ʪ�����ɫʯ����ֽ���ɫ���������ɫʯ����ֽ�����Ա仯����Ӧ�����ű���ʽ��H2O+CO2![]() H2 CO3��3������Y�ε��̶ܹ�������̨�ϣ�a ��λ���Ϸ���b ��λ���·�������ʯ����ֽ��ʪ�۲쵽b������ֽ��ɫ��a �����ԣ�ԭ��������̼���ܶȱȿ����������³�
H2 CO3��3������Y�ε��̶ܹ�������̨�ϣ�a ��λ���Ϸ���b ��λ���·�������ʯ����ֽ��ʪ�۲쵽b������ֽ��ɫ��a �����ԣ�ԭ��������̼���ܶȱȿ����������³�