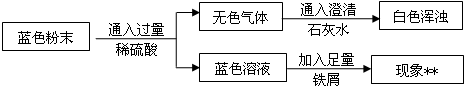
��Ŀ����
ij�о���ѧϰС��ԡ�SO2�ܷ���H2O��Ӧ�����ᡱ����̽��������������ǵ�̽��������ش��й����⡣
��1���������ϣ���SO2��������һ����ɫ���壬������ˮ��������ʹ��ɫʯ����ֽ��ɺ�ɫ����SO2�ж���
��2��������裺SO2����H2O��Ӧ�����ᡣ
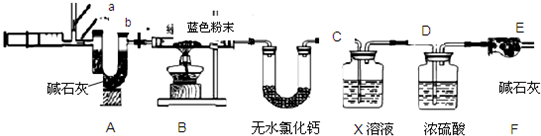
��3��ʵ��̽�����������ͼ��ʾװ�ý���ʵ�顣
��ʵ������У�Aװ������ɫʯ����ֽ����ɫʼ��û�б仯��Aװ�õ�����___________
_______��
����ͨ��SO2֮ǰ��Bװ���н�ͷ�ι��ڵ�����ˮ�ε���ɫʯ����ֽ�ϣ�δ����ֽ��ɫ�����仯���˲�������Ŀ����______________________________________________________������SO2ͨ��ʱ����ʪ�����ɫʯ����ֽ��죬������˵��___________________________���˹����з�Ӧ�Ļ�ѧ����ʽΪ____________________________________________________��
��4�����ۣ�ԭ���������
��5����˼�����ۣ���ʵ�鷽���У���һ�����Ե���©�����������ָ������֮��____________
______________________________________________________________________________��
��6����չ̽�������о���ѧϰС��ȡ�ս������᳧�����������в���SO2����������ˮ���вⶨ��ÿ�������Ӳ�һ��pH�����������±���ʾ��
| �ⶨʱ�� | 5��05 | 5��10 | 5��15 | 5��20 | 5��25 | 5��30 | 5��35 |
| pH | 4.95 | 4.94 | 4.94 | 4.88 | 4.86 | 4.85 | 4.85 |
�����������ݵı仯����ɵó��Ľ�����_____________________________����Դ˽��ۣ���²����е�ԭ��Ϊ________________________________________����ϴ���ʯ�����ܵ����긯ʴ����ʵ������Ϊ̼�ᡢ���ᡢ�����ᣨH2SO3��������ǿ������˳����___________
_________________________________________________________��
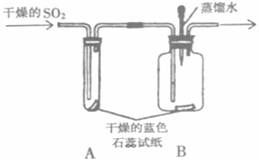
��3����֤��SO2����ʹ��ɫʯ����ֽ��ɫ
��֤��ˮ����ʹ��ɫʯ����ֽ��ɫ����֤��ˮû�����ԣ� SO2��ˮ��Ӧ������
SO2+H2O=H2SO3
��5��β��û�д�������Ӧ�ӽ�һ��β������װ�ã�
��6������ʱ��ı仯����ˮ����������ǿ H2SO3������е�O2��Ӧ������H2SO4 H2SO4>H2SO3>H2CO3

 ����С����ͬ������ϵ�д�
����С����ͬ������ϵ�д�