��Ŀ����
Ϊ�˲ⶨijƷ��ʳ�ô�����̼���Ƶ�����������ijУ��ѧ�о���ѧϰС���̽���������£�
[�������]��Ʒ��̼���Ƶ����������Ƕ��٣�
[֪ʶ��]
ʳ�ô������Ҫ�ɷ���̼���ƣ���������������Ȼ��ƣ���Ӧ�����в�����ˮ���Ȼ���Ļӷ���
[��Ʒ�����ʵ��]
����ͬѧ����ȡ12.00��Ʒ����ˮ�����Һ������Һ�м����������ʯ��ˮ�����ˡ�ϴ�ӡ�������õ���ɫ����10.00g��
����ͬѧ����ȡ12.00��Ʒ������������ϡ����ֱ����Ӧֹͣ�����ռ���4.4g������̼��
[�������]
������ѡһ��ͬѧ��ʵ�������������Ǽ������Ʒ��̼���Ƶ�������______��̼���Ƶ�����������______������������ȷ��0.1%��
[������˼]
��1�������С��ͬѧ��Ϊ��Ҫ���̼���Ƶ�������Ҳ����ʹ���������ʯ��ˮ�������ͬ����������______����һ�־������ʵĻ�ѧʽ������Һ����Ʒ��Ӧ��ͨ���ⶨ������ʵ������������йؼ��㼴�ɣ�
��2�������С��ͬѧ��Ϊ������ϡ�����������������Ҳ�������ȡ13.5g��Ʒ�����ձ��У�ÿ�μ���20gϡ���ᣨ������ˮ���Ȼ����ݳ������þ���������������¼ʵ���������£�
��������a=______g��b=______g�����������±ߵ�����ֽ�ϻ����������������������ϡ����������ϵ�����ߣ�
[�������]��Ʒ��̼���Ƶ����������Ƕ��٣�
[֪ʶ��]
ʳ�ô������Ҫ�ɷ���̼���ƣ���������������Ȼ��ƣ���Ӧ�����в�����ˮ���Ȼ���Ļӷ���
[��Ʒ�����ʵ��]
����ͬѧ����ȡ12.00��Ʒ����ˮ�����Һ������Һ�м����������ʯ��ˮ�����ˡ�ϴ�ӡ�������õ���ɫ����10.00g��
����ͬѧ����ȡ12.00��Ʒ������������ϡ����ֱ����Ӧֹͣ�����ռ���4.4g������̼��
[�������]
������ѡһ��ͬѧ��ʵ�������������Ǽ������Ʒ��̼���Ƶ�������______��̼���Ƶ�����������______������������ȷ��0.1%��
[������˼]
��1�������С��ͬѧ��Ϊ��Ҫ���̼���Ƶ�������Ҳ����ʹ���������ʯ��ˮ�������ͬ����������______����һ�־������ʵĻ�ѧʽ������Һ����Ʒ��Ӧ��ͨ���ⶨ������ʵ������������йؼ��㼴�ɣ�
��2�������С��ͬѧ��Ϊ������ϡ�����������������Ҳ�������ȡ13.5g��Ʒ�����ձ��У�ÿ�μ���20gϡ���ᣨ������ˮ���Ȼ����ݳ������þ���������������¼ʵ���������£�
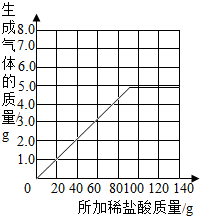
| ��������Ĵ��� | 1 | 2 | 3 | 4 | 5 | 6 | 7 |
| �ձ�����������������/g | 78.9 | 97.8 | 116.7 | 135.60 | 155.05 | 175.05 | 195.05 |
| �������������/g | 1.1 | 2.2 | a | 4.4 | 4.95 | b | -- |

������10g̼�������̼���Ƶ�����Ϊx������
Na2CO3+Ca��OH��2�TCaCO3��+2NaOH
106 100
x 10g
=
x=10.6g
̼���Ƶ����������ǣ�
��100%=88.3%��
��1��̼���ƻ�����ijЩ���п����Ը����ӻ����ӵ��ν�����ɳ��������Ա����Ϊ��CaCl2��
��2�����������������ݿ��Է��֣�ÿ����20g���������ɶ�����̼������Ϊ1.1g���ʵ�����ʱ�����Ķ�����̼������Ϊ3.3g������Ʋ⣬������ʱ������̼������ӦΪ5.5g������ֻ��4.95g��˵��̼����ȫ���μӷ�Ӧ���ʵ�����ʱ�ټ���������ж�����̼������Ҫ�������ߣ���Ҫ���յ㣬�ɱ����������ݿ���֪����̼�����ټ����������Ϊ80g��100g֮��ʱȫ���μӷ�Ӧ��������ɶ�����̼������Ϊ4.95g�����Ա����Ϊ��3.3��4.95��
Na2CO3+Ca��OH��2�TCaCO3��+2NaOH
106 100
x 10g
| 106 |
| x |
| 100 |
| 10g |
x=10.6g
̼���Ƶ����������ǣ�
| 10.6g |
| 12g |
��1��̼���ƻ�����ijЩ���п����Ը����ӻ����ӵ��ν�����ɳ��������Ա����Ϊ��CaCl2��
��2�����������������ݿ��Է��֣�ÿ����20g���������ɶ�����̼������Ϊ1.1g���ʵ�����ʱ�����Ķ�����̼������Ϊ3.3g������Ʋ⣬������ʱ������̼������ӦΪ5.5g������ֻ��4.95g��˵��̼����ȫ���μӷ�Ӧ���ʵ�����ʱ�ټ���������ж�����̼������Ҫ�������ߣ���Ҫ���յ㣬�ɱ����������ݿ���֪����̼�����ټ����������Ϊ80g��100g֮��ʱȫ���μӷ�Ӧ��������ɶ�����̼������Ϊ4.95g�����Ա����Ϊ��3.3��4.95��


��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ




