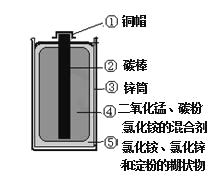
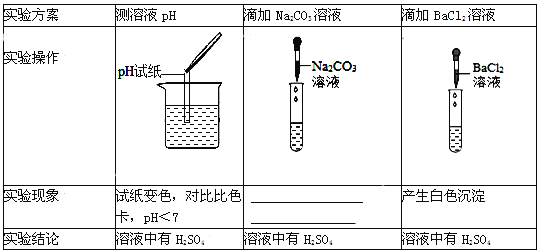
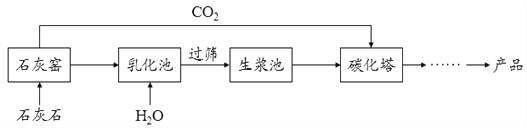
��Ŀ����
����Ŀ����������(FeSO4)����������Ѫ�����̷�(FeSO4��7H2O)����һ����Ҫ��ʳƷ���������Ӽ���ʵ����������ϡ���ᷴӦ�ȿ����Ƶ�����������Ҳ�����Ƶ��̷���
(1)����7.6g������������Ԫ�ص�����___________��
(2)�Ķ��±ߵ���Ϣ��ʾ����ʽ����FeSO4��7H2O����Է�������__________��

(3)��6g����������װ������ϡ������ձ��У���ַ�Ӧ���ձ������ʵ�������������0.2g�����㣺
��������������������_________��
��������������������____________��
���𰸡� 2.8g (56+32+16��4) +7��(1��2+16)��278 15.2 93.3%
�����������⿼������Է��������ĸ������������ݻ�ѧ��Ӧ����ʽ�ļ�������������ijԪ�ص���������
��1��7.6��������������Ԫ�ص�����Ϊ��7.6g��![]() ��100%=2.8g��
��100%=2.8g��
��2��FeSO47H2O����Է�������Ϊ����56+32+16��4��+7��(1��2+16��=278��
��3��������������Ϊx��������������������Ϊy��
Fe+H2SO4�TFeSO4+H2��
56 152 2
x y 0.2g
![]() x=5.6g��
x=5.6g��
![]() y=15.2g��
y=15.2g��
����������������������![]() ��100%=93.3%��
��100%=93.3%��
����1��7.6��������������Ԫ�ص�����Ϊ2.8g��
��2��FeSO47H2O����Է�������Ϊ����56+32+16��4��+7��(1��2+16��=278��
��3��������15.2g����������
��4��������������������Ϊ93.3%��

����Ŀ����ͼΪ���������ֻ���ʵ�飬�ɴ������ɼ�ʵ�������Ϣ�������ߣ���������X��ʾ���������̼��Ӧ��ijҺ�壬������Y��ʾ�ձ��е�ij���������ж�����X��������Y�ķ�����ȷ��
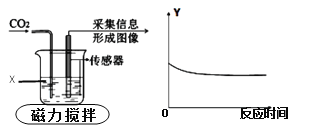
ѡ�� | ����X | ������Y |
A | ˮ | ��Һ������ |
B | ˮ | ��Һ��pH |
C | ����������Һ | ���ʵ����� |
D | ����������Һ | ��Һ�и�Ԫ������ |
A. A B. B C. C D. D