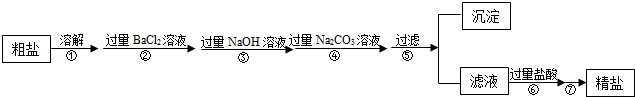
题目内容
过氧化钠(化学式为Na2O2)是一种淡黄色的固体物质,它能与水发生化学反应,其反应的化学方程式是2Na2O2+2H2O=4NaOH+O2↑,现将一定质量的过氧化钠加入到盛有175.2g氢氧化钠溶液的烧杯中,反应完毕后称得溶液的质量比反应前过氧化钠和水的总质量减少了6.4g,所得溶液中溶质的质量分数为20%,试计算:
(1)反应生成氧气的质量是 g。
(2)烧杯中的氢氧化钠溶液中溶质的质量分数。
(1)反应生成氧气的质量是 g。
(2)烧杯中的氢氧化钠溶液中溶质的质量分数。
(1)6.4g;(2)4.6%
试题分析:(1)反应完毕后称得溶液的质量比反应前过氧化钠和水的总质量减少了6.4g,所以反应产生的氧气质量为6.4g。
(2)设参加反应的过氧化钠的质量为
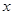 ,生成的氢氧化钠的质量为
,生成的氢氧化钠的质量为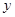 。
。2Na2O2 + 2H2O =" 4NaOH" + O2↑
156 160 64
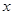
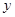 6.4g
6.4g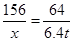 ,解得
,解得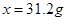
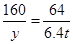 ,解得
,解得

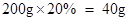

答:烧杯中的氢氧化钠溶液中溶质的质量分数为4.6%。
点评:这类题目通常难在审题和计算上,同时对基础知识有一定的要求,要求考生平时注意牢牢掌握基础知识,同时训练自己的计算技能。

练习册系列答案
 阅读快车系列答案
阅读快车系列答案
相关题目