��Ŀ����
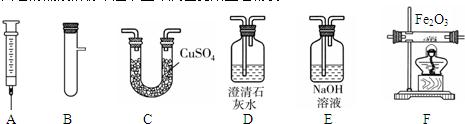
��6�֣�ijͬѧ����ͼ��ʾװ����ȡ�����������CO�����ⶨij����ͭ��ͭ�Ļ������ͭ���ʵĺ�����
����
�ٳ����£����ᣨHCOOH������ɫ�ӷ���Һ�壬��Ũ�����������ֽ⡣
����ֽ�Ļ�ѧ����ʽΪ��HCOOH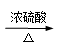 CO��+ H2O��
CO��+ H2O��
��Ũ���������ˮ�ԣ��ѻӷ����������������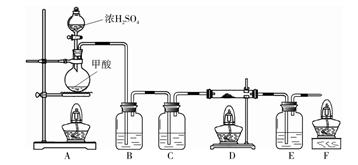
��1��E�г���ʯ��ˮ����ǣ���Ӧ�Ļ�ѧ����ʽ�� ��
��2��װ��B�������� ��װ��C�е��Լ��� ����ȼװ��F�оƾ��Ƶ�Ŀ����
��
��3��ʵ�����ʱ��Ϩ��ƾ��Ƶ�˳���� �����Ӧװ����ţ���
��4������ȡ�û����5.0 g����ַ�Ӧ��������ʣ����������Ϊ4.8 g��ԭ�������ͭ���ʵ���������Ϊ ��
��1��CO2 + Ca(OH) 2 = CaCO3��+ H2O
��2����ȥ�ӷ������ļ��� Ũ���� ����β������ֹ������Ⱦ
��3��DAF ��4��80%
���������������1��E�г���ʯ��ˮ����ǣ��Ƕ�����̼���������Ʒ�Ӧ�ˣ���Ӧ�Ļ�ѧ����ʽ��CO2 + Ca(OH) 2 = CaCO3��+ H2O��
��2��װ��B�������dz�ȥ�ӷ������ļ��ᣬװ��C�е��Լ���Ũ���ᣬ�������塣��ȼװ��F�оƾ��Ƶ�Ŀ���Ǵ���β������ֹ������Ⱦ��
��3��ʵ�����ʱ��Ϩ��ƾ��Ƶ�˳����DAF����������ͭ�ķ�Ӧֹͣ����ֹͣ����ȣ�����ʱ����ͨ��һ����̼��������ȴ����ֹͣͨ��һ����̼������һ����̼�ж��������Ϩ��F�ƾ��ƣ�������ȥһ����̼����ֹ��Ⱦ������
��4������ȡ�û����5.0 g����ַ�Ӧ��������ʣ����������Ϊ4.8 g������5.0-4.8=0.2g��Ԫ����ʧ����ԭ�������ͭ���ʵ���������Ϊx%������5.0����1- x%����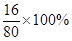 =0.2g��
=0.2g��
x%=80%��
���㣺������̼�����ʣ���ѧ����ʽ��һ����̼�����ʣ�ʵ�������̽������������Ԫ�ص�����������
������������̼��ʹ����ʯ��ˮ����ǣ�һ����̼�ж���
ijԪ�ص���������=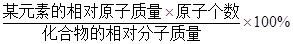 ��
��

 ��һ������ĩ�ٷֳ�̾�ϵ�д�
��һ������ĩ�ٷֳ�̾�ϵ�д�



 CO��+ H2O��
CO��+ H2O��