��Ŀ����
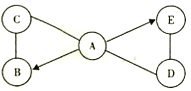
����Ŀ����������ĸ��ʾ���������ʾ���H��C��O��Fe�еļ���Ԫ����ɡ�
(1)A��B����ͬԪ�������A�������к�������������д��B�ֽ�ΪA�Ļ�ѧ����ʽ��_______________��
(2)D��E�ڳ����¾�Ϊ��̬������������D������ȼ����д��Dת��ΪE�Ļ�ѧ����ʽ��_______________��
(3)M��N����ͬԪ�������M�Ļ�ѧʽΪFeaOb(a��b��Ϊ����)������Է�������Ϊ160����a��bΪ____����һ�������¿��Է������з�Ӧ��N+4D=3Fe+4E����N�Ļ�ѧʽΪ___��
���𰸡�2H2O2![]() 2H2O+O2��2CO+O2
2H2O+O2��2CO+O2![]() 2CO22��3Fe3O4
2CO22��3Fe3O4
��������
(1)���庬��������ˮ��A��B������ͬԪ����ɣ�����B�ǹ������⣬���������ڶ������̵Ĵ�����������ˮ����������ѧ����ʽΪ��2H2O2![]() 2H2O+O2����
2H2O+O2����
(2)D������ȼ�ϣ�D��E�ڳ����¾�Ϊ��̬���������D����һ����̼��E���Ƕ�����̼��һ����̼�������ڵ�ȼ�����������ɶ�����̼����ѧ����ʽΪ��2CO+O2![]() 2CO2��
2CO2��
(3)�������ԭ��������56���������ԭ��������16������56a+16b=160�������7a+2b=20��a��bΪ����������a=2��b=3��N+4CO=3Fe+4CO2������N����������ԭ�Ӻ��ĸ���ԭ�ӣ���N�Ļ�ѧʽΪ��Fe3O4��

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�����Ŀ����һ���ܱ���������������������̼��ˮ������һ��δ֪����M����һ�������³�ַ�Ӧ����÷�Ӧǰ������ʵ��������±���
���� | ���� | ������̼ | ˮ���� | M |
��Ӧǰ����/g | 100 | 1 | 1 | 46 |
��Ӧ������/g | 4 | 89 | 55 | x |
(1)���������غ㶨�ɣ�����Ϊx��ֵӦΪ_____________��
(2)δ֪����Mһ�����е�Ԫ��Ϊ______________________��
(3)��֪δ֪����M����Է�������Ϊ46���Ƴ��仯ѧʽΪ_____________��
(4)�÷�Ӧ�Ļ�ѧ����ʽΪ______________________________��