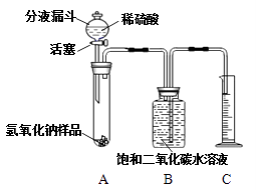
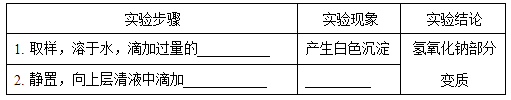
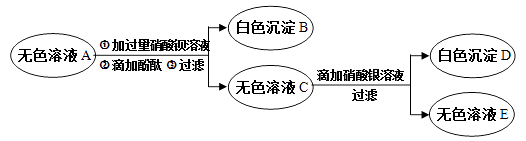
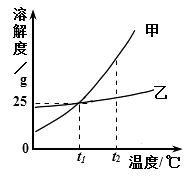
��Ŀ����
����Ŀ������֪������������ʯ��ʯ����Ҫ�ɷ���̼��ƣ����Ƶ���ʯ�ң��������ƣ��Ͷ�����̼��Ϊ�˷�����Ʒ�Ĵ��ȣ�С˴ͬѧȡʯ��ʯ��Ʒ22g��������պ�Ƶ�ʣ����������Ϊ13.2g����ʾ���ٶ����ʲ���Ӧ����ѧ����ʽCaCO3 ![]() CaO+CO2���������㣺
CaO+CO2���������㣺
��1�����ɶ�����̼�������Ƕ��ٿˣ���Щ������̼�ڱ�״���µ�����Ƕ�����������״����CO2���ܶ�1.977g/L��
��2���Ƶ���ʯ�ҵ������Ƕ��ٿˣ�
���𰸡�
��1�����ɶ�����̼������Ϊ��22g��13.2g=8.8g�������ܶȵĹ�ʽ��������̼�����Ϊ��8.8g��1.977g/L=4.45L
��2���⣺��������ʯ�ҵ�����Ϊx
CaCO3 | CO2�� |
56 | 44 |
x | 8.8g |
56:44=x��8.8g
X=11.2g
���Ƶ���ʯ�ҵ�������11.2g��
����������1�����������غ㶨�ɿ�֪����ѧ��Ӧǰ�����ʵ����������䣬�����ɶ�����̼������Ϊ��22g��13.2g=8.8g�������ܶȵĹ�ʽ��������̼�����Ϊ��8.8g��1.977g/L=4.45L��2�����ݻ�ѧ����ʽ��CaCO3 ![]() CaO+CO2����CaO��CO2��������ϵ����������Ƶ���ʯ�ҵ�������
CaO+CO2����CaO��CO2��������ϵ����������Ƶ���ʯ�ҵ�������
�ʴ�Ϊ�����ɶ�����̼������Ϊ��22g��13.2g=8.8g�������ܶȵĹ�ʽ��������̼�����Ϊ��8.8g��1.977g/L=4.45L��
�⣺��������ʯ�ҵ�����Ϊx
CaCO3 | CO2�� |
56 | 44 |
x | 8.8g |
56:44=x��8.8g
X=11.2g
���Ƶ���ʯ�ҵ�������11.2g��
������Ҫ���ݻ�ѧ����ʽ�ļ�����м��㣬���μӷ�Ӧ����������֮�ȵ�����Է����������Ի�ѧ������֮�ȡ�

����Ŀ�����й����ȷ����
ѡ �� | �� �� | ���� |
A | ��ʯȼ�� | ú��ʯ�͡���Ȼ�� |
B | Ӫ������ | ���ࡢ��֬�������� |
C | �����ļ� | ��������ơ���ʯ�� |
D | �ϳɲ��� | ����ϩ�����ڡ��ϳ��� |
A.AB.BC.CD.D