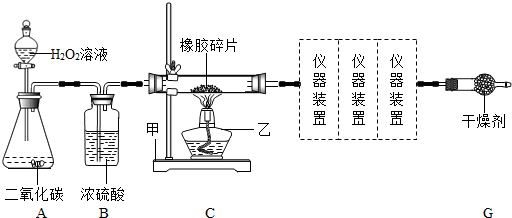
��Ŀ����
�����������࣮����Ȼ������C��HԪ�أ��м�����Ԫ�غ�õ�����������������̥��Ь�ij��ò��ϣ�ij��ȤС���ͬѧ������Ƭ��Ʒ����Ԫ�ؽ���ʵ��̽����ͨ���������ϻ�õļ��鼸������������Ϣ���±����������̽��������ͨ��ʵ��ó����ۣ�
| ���� | �Լ� | ���� | ��ע |
| ˮ���� | ��ˮ����ͭ����ɫ���壩 | ��ɫ��������ɫ | �������� ������ |
| �������� | Ʒ����Һ����ɫ�� | ��Һ�ĺ�ɫ��ȥ |
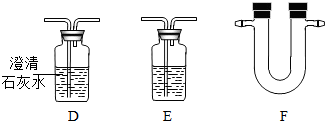
�������߿��ڵ�����װ�úͿ����õ����Լ����±���
| ����װ�� | �Լ� |
 | Ũ���ᡢ����ʯ��ˮ�� ��ˮ����ͭ����ɫ���壩�� Ʒ����Һ���������ƹ��壮 |
��1����װ��C�У�������������______�������ҵ���;��______��װ��B�е�Ũ�����������______��װ��A�����ɵ��������ƻ�ѧʽ��______����Ӧ�Ļ�ѧ����ʽΪ______��
��2��ѡ��װ��A��������ȡ�������壬����һ������Ļ�ѧʽ��______��
��3������ȤС���ͬѧͨ��ʵ��ó�����Ʒ�к���C��H��S����Ԫ�صĽ��ۣ����ǵ���������װ������˳����A��B��C��______��______��______��G������ţ�������װ��F����ʢ�Լ�Ϊ______�����������ƻ�ѧʽ����
��4������ȤС���ijͬѧ��ʵ���з��֣�װ��C�в������ڱ���������ɫ������֣�����Ϊ��װ��C�ķ�Ӧ�л�������һ������δ���������Ϊ��������______�����������ƻ�ѧʽ����
�⣺��1��������Ϊ����̨����װ��Ϊ�ƾ��ƣ������������ʼ��ȣ���ΪAװ���ڷ�Ӧ���������Ĺ����л����һ����ˮ��������װ��B�е�Ũ����������ˮ������ֹ����ʵ��������д��Aװ���з�Ӧ�Ļ�ѧ����ʽ��
��2��װ��A���ڳ����·�Ӧ��ȡ����ģ���Ӧ���״̬Ϊ�����Һ�壬���Ը�װ�û�����������ȡ������̼���壻
��3�����������װ�ÿ���֪��������Ҫ��֤�������У�������̼�����������ˮ��Ϊ�˷�ֹ����ͨ��ʯ��ˮ��Ʒ��ʱ������ˮ��������ʵ����������Ӧ����ͨ��Fװ�������ղ���֤ˮ�Ĵ��ڣ�����Fװ���м��������Ϊ��ˮ����ͭ����������Ҳ����ʹ����ʯ��ˮ����ǣ�����Ӧ������֤����������ͨ������ʯ��ˮ��֤������̼���ʿ����жϳ�װ�õ�����˳��
��4�������ijɷֿ���֪���ú�ɫ�������Ϊû���ü���Ӧ��̼���ʣ���������̼���Ժ�̼��Ӧ����һ����̼�������������Ϊһ����̼���壮
�ʴ�Ϊ����1������̨�������ʼ��ȣ�����ˮ�֣���ֹ����ʵ������������2H2O2 2H2O+O2����
2H2O+O2����
��2��������̼��
��3��F��E��D����ˮ����ͭ��
��4��һ����̼��
��������1��ֱ��д�������������ƣ������������Ϣ�����ѧ֪ʶ���ж�������ҩƷ����;������д��ѧ����ʽ��
��2������װ��A���ص㼰�����÷�Χ�����
��3����������������ϲ������ж϶�װ�ý��к����İ��ţ�����գ�
��4�������������֪���ú�ɫ�������Ϊ̼�����Կ��Ծݴ��жϿ������ɵ����壮
������֪���������ʵļ��������ܹ�����ʵ��Ҫ��ѡ����ʵļ���˳���ڽ��������Ŀʱע������IJ������ϲ��֣�������ǵĽ�����нϴ�İ�����
��2��װ��A���ڳ����·�Ӧ��ȡ����ģ���Ӧ���״̬Ϊ�����Һ�壬���Ը�װ�û�����������ȡ������̼���壻
��3�����������װ�ÿ���֪��������Ҫ��֤�������У�������̼�����������ˮ��Ϊ�˷�ֹ����ͨ��ʯ��ˮ��Ʒ��ʱ������ˮ��������ʵ����������Ӧ����ͨ��Fװ�������ղ���֤ˮ�Ĵ��ڣ�����Fװ���м��������Ϊ��ˮ����ͭ����������Ҳ����ʹ����ʯ��ˮ����ǣ�����Ӧ������֤����������ͨ������ʯ��ˮ��֤������̼���ʿ����жϳ�װ�õ�����˳��
��4�������ijɷֿ���֪���ú�ɫ�������Ϊû���ü���Ӧ��̼���ʣ���������̼���Ժ�̼��Ӧ����һ����̼�������������Ϊһ����̼���壮
�ʴ�Ϊ����1������̨�������ʼ��ȣ�����ˮ�֣���ֹ����ʵ������������2H2O2
 2H2O+O2����
2H2O+O2������2��������̼��
��3��F��E��D����ˮ����ͭ��
��4��һ����̼��
��������1��ֱ��д�������������ƣ������������Ϣ�����ѧ֪ʶ���ж�������ҩƷ����;������д��ѧ����ʽ��
��2������װ��A���ص㼰�����÷�Χ�����
��3����������������ϲ������ж϶�װ�ý��к����İ��ţ�����գ�
��4�������������֪���ú�ɫ�������Ϊ̼�����Կ��Ծݴ��жϿ������ɵ����壮
������֪���������ʵļ��������ܹ�����ʵ��Ҫ��ѡ����ʵļ���˳���ڽ��������Ŀʱע������IJ������ϲ��֣�������ǵĽ�����нϴ�İ�����

��ϰ��ϵ�д�
�����Ŀ
�����������࣮����Ȼ������C��HԪ�أ��м�����Ԫ�غ�õ�����������������̥��Ь�ij��ò��ϣ�ij��ȤС���ͬѧ������Ƭ��Ʒ����Ԫ�ؽ���ʵ��̽����ͨ���������ϻ�õļ��鼸������������Ϣ���±����������̽��������ͨ��ʵ��ó����ۣ�
��ͼΪ����ʵ��װ�õ�һ���֣�
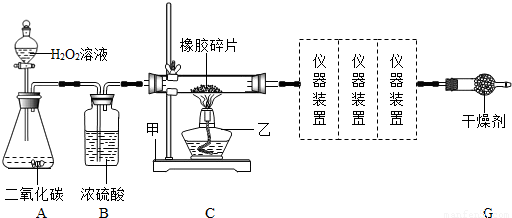
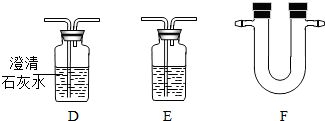
�������߿��ڵ�����װ�úͿ����õ����Լ����±���
��ش��������⣨�������߿��ڸ�����װ���о���ַ�Ӧ����
��1����װ��C�У�������������______�������ҵ���;��______��װ��B�е�Ũ�����������______��װ��A�����ɵ��������ƻ�ѧʽ��______����Ӧ�Ļ�ѧ����ʽΪ______ 2H2O+O2��
| ���� | �Լ� | ���� | ��ע |
| ˮ���� | ��ˮ����ͭ����ɫ���壩 | ��ɫ��������ɫ | �������� ������ |
| �������� | Ʒ����Һ����ɫ�� | ��Һ�ĺ�ɫ��ȥ |

�������߿��ڵ�����װ�úͿ����õ����Լ����±���
| ����װ�� | �Լ� |
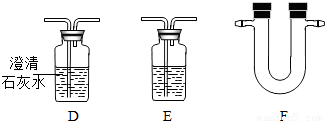 | Ũ���ᡢ����ʯ��ˮ�� ��ˮ����ͭ����ɫ���壩�� Ʒ����Һ���������ƹ��壮 |
��1����װ��C�У�������������______�������ҵ���;��______��װ��B�е�Ũ�����������______��װ��A�����ɵ��������ƻ�ѧʽ��______����Ӧ�Ļ�ѧ����ʽΪ______ 2H2O+O2��