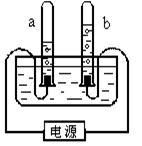
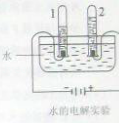
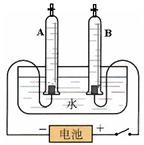
��Ŀ����
ijУ����С���ͬѧ������ˮ�ĵ��ʵ��̽��ˮ����ɣ�������ȡ192.7mLˮ��ˮ���ܶ�Ϊ1.00g/cm3��������ˮ�м�����7.3g�������ƹ��壬����ܽ����ͼ��ʾ��װ�ý���ʵ�飬��ͨ��Դ����A���ռ���22.3mL���壨�����ܶ�Ϊ0.09g/L��ʱ��ֹͣʵ�顣������ش�
��1��ˮͨ��ֽ�Ļ�ѧ��Ӧ����ʽΪ��
��2��ʵ����A�����ɵ����������� ��
��3����ˮ�м����������ƹ����Ŀ����
��
��4��ֹͣʵ��ʱʣ�ࡰˮ�С��������Ƶ����������Ƕ��٣�����ʽ���㣩

��1��ˮͨ��ֽ�Ļ�ѧ��Ӧ����ʽΪ��
��2��ʵ����A�����ɵ����������� ��
��3����ˮ�м����������ƹ����Ŀ����
��
��4��ֹͣʵ��ʱʣ�ࡰˮ�С��������Ƶ����������Ƕ��٣�����ʽ���㣩
(1)2H 2O 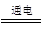 2H2��+O2��?
2H2��+O2��?
(2)22.3mL��0.09g/L��10-3=0.002g?
(3)��ǿ��ˮ���ĵ�����?
(4) ��100%=3.65% ��2�֣�
��100%=3.65% ��2�֣�
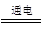 2H2��+O2��?
2H2��+O2��?(2)22.3mL��0.09g/L��10-3=0.002g?
(3)��ǿ��ˮ���ĵ�����?
(4)
 ��100%=3.65% ��2�֣�
��100%=3.65% ��2�֣���1�����ˮ�Ļ�ѧ����ʽΪ2H 2O 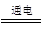 2H2��+O2������2��m=��V=22.3mL��0.09g/L��10-3="0.002g;" ��3��������ˮ�Dz�����ģ�����NaOH����Ŀ�ľ�����ǿ��ˮ���ĵ����ԣ���4��������0.002gH2��Ҫˮ������Ϊ 0.002g ��9=0.018g����Ӧ����Һ������=192.7mL��1.00g/cm3+7.3g-0.018g=199.982g����NaOH����������Ϊ
2H2��+O2������2��m=��V=22.3mL��0.09g/L��10-3="0.002g;" ��3��������ˮ�Dz�����ģ�����NaOH����Ŀ�ľ�����ǿ��ˮ���ĵ����ԣ���4��������0.002gH2��Ҫˮ������Ϊ 0.002g ��9=0.018g����Ӧ����Һ������=192.7mL��1.00g/cm3+7.3g-0.018g=199.982g����NaOH����������Ϊ
7.3g��199.982g��100%="3.65%" ��
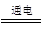 2H2��+O2������2��m=��V=22.3mL��0.09g/L��10-3="0.002g;" ��3��������ˮ�Dz�����ģ�����NaOH����Ŀ�ľ�����ǿ��ˮ���ĵ����ԣ���4��������0.002gH2��Ҫˮ������Ϊ 0.002g ��9=0.018g����Ӧ����Һ������=192.7mL��1.00g/cm3+7.3g-0.018g=199.982g����NaOH����������Ϊ
2H2��+O2������2��m=��V=22.3mL��0.09g/L��10-3="0.002g;" ��3��������ˮ�Dz�����ģ�����NaOH����Ŀ�ľ�����ǿ��ˮ���ĵ����ԣ���4��������0.002gH2��Ҫˮ������Ϊ 0.002g ��9=0.018g����Ӧ����Һ������=192.7mL��1.00g/cm3+7.3g-0.018g=199.982g����NaOH����������Ϊ7.3g��199.982g��100%="3.65%" ��

��ϰ��ϵ�д�
 ��Уͨ��֤��Ч��ҵϵ�д�
��Уͨ��֤��Ч��ҵϵ�д�
�����Ŀ