��Ŀ����
��3�֣���һ��ʯ��ʯ��Ʒ�ijɷ���CaCO3��SiO2����֪SiO2�Ȳ�����ˮҲ�������ᷴӦ�����ֽ�һ������ʯ��ʯ��Ʒ�����ձ��У��ٽ�100gϡ�����4�μ����ձ��У�ÿ�ξ���ַ�Ӧ��ʵ�����ݼ�¼���£�
��1������������M��ֵΪ__________
��2����ʯ��ʯ��Ʒ��̼��Ƶ�����������
��3�����ɶ�����̼����������
| | ��һ�� | �ڶ��� | ������ | ���Ĵ� |
| ����ϡ���������/g | 25 | 25 | 25 | 25 |
| ��ַ�Ӧ��ʣ���������/g | 7 | 4 | 2 | M |
��2����ʯ��ʯ��Ʒ��̼��Ƶ�����������
��3�����ɶ�����̼����������
2g 80% 3.52g
������������⡿���������֪��ÿ����25gϡ���ᣬ���彫��Ӧ��3g���������η�Ӧ����廹��2g��˵����ʱ�����е�2g�����Ƕ������裬�������Ӧ�ˡ��ʣ�
��1������������M��ֵΪ2g ��
��2���������ݱ��ɷ���25��ϡ������ȫ��ӦӦ����̼���7 g - 4 g ="3" g����˷�Ӧǰʯ��ʯ��Ʒ������ӦΪ7 g +3 g ="10" g��
��Ʒ��CaCO3����������Ϊ��
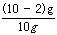 ��100% ��80%
��100% ��80% ��3���裺���ɶ�����̼������Ϊx��
CaCO3 + 2HCl
 CaCl2 + H2O + CO2��
CaCl2 + H2O + CO2��100 44
8g x
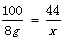
x=3.52g
�����ɶ�����̼������Ϊ3.52g��
���������ݻ�ѧ����ʽ���㣬Ҫע�����IJ��裬�衢д���ҡ��С��⡢�𡣱����з������ݣ��ó�ÿ����25gϡ���ᣬ���彫��Ӧ��3g��2g�����Ƕ��������ǽ���Ĺؼ���

��ϰ��ϵ�д�
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д�
�����Ŀ




 2N2+3H2O������Ӧ��õ�28 gN2�����������İ������ٿˣ�
2N2+3H2O������Ӧ��õ�28 gN2�����������İ������ٿˣ� Ca(OH)2��C2H2������Ҫ��ȡ13 g��Ȳ���壬��Ҫ̼���Ƶ������Ƕ��ٿˣ�
Ca(OH)2��C2H2������Ҫ��ȡ13 g��Ȳ���壬��Ҫ̼���Ƶ������Ƕ��ٿˣ�