��Ŀ����
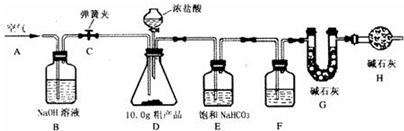
19����֪ij����ֲ�Ʒ������NaCl���ʣ�Ϊ�ⶨ�ô���ֲ�Ʒ�д��������������ij��ȤС���ͬѧ�������ͼ��ʾ��ʵ��װ�ã�ȡ10.0g�ֲ�Ʒ����ʵ�飮

˵������ʯ����CaO��NaOH �Ĺ������Eװ���еı���NaHCO3��Һ��Ϊ�˳�ȥ������̼�����е��Ȼ��⣬�����ķ�ӦΪNaHCO3ʮHC1=NaClʮCO2��ʮH2O��
ʵ����Ҫ�����������£�
�����Ӻ�װ�ã���������ԣ��ڴ��ɼ�C����A������ͨ��һ��ʱ��������۳��� G���������ܹرյ��ɼ�C�������μ�Ũ������������ֱ��D��������ð�����ݴ��ɼ�C���ٴλ���ͨ��һ��ʱ����������ٴγ��� G����������ǰ������������Ϊ0.48g��
��ش��������⣺
��1������������ƽ������Ʒʱ�������ƽ��ָ������ƫת��˵��
��2��F�е��Լ�ӦΪ
��3��Bװ�õ�������
��4��Hװ�õ�������
��5����ʵ��10.0g�ֲ�Ʒֻ�ܲ���0.44g CO2��������ϸ��������ʵ�飬����ʵ��ֵ

˵������ʯ����CaO��NaOH �Ĺ������Eװ���еı���NaHCO3��Һ��Ϊ�˳�ȥ������̼�����е��Ȼ��⣬�����ķ�ӦΪNaHCO3ʮHC1=NaClʮCO2��ʮH2O��
ʵ����Ҫ�����������£�
�����Ӻ�װ�ã���������ԣ��ڴ��ɼ�C����A������ͨ��һ��ʱ��������۳��� G���������ܹرյ��ɼ�C�������μ�Ũ������������ֱ��D��������ð�����ݴ��ɼ�C���ٴλ���ͨ��һ��ʱ����������ٴγ��� G����������ǰ������������Ϊ0.48g��
��ش��������⣺
��1������������ƽ������Ʒʱ�������ƽ��ָ������ƫת��˵��
��Ʒ�أ�������
����2��F�е��Լ�ӦΪ
Ũ����
����3��Bװ�õ�������
��ȥ�����еĶ�����̼
��Bװ���з�����Ӧ�Ļ�ѧ����ʽΪ��CO2+2NaOH=Na2CO3+H2O
����4��Hװ�õ�������
��ֹ�����еĶ�����̼��ˮ������װ��G�м�ʯ������
����5����ʵ��10.0g�ֲ�Ʒֻ�ܲ���0.44g CO2��������ϸ��������ʵ�飬����ʵ��ֵ
��������1����ʹ����ƽ���г���ʱ������ʹ��ԭ���������롱��ҩƷӦ������ƽ�����̣���ƽ��ָ��ƫ����ߣ�˵�����ҩƷ�����������룻
��2��Ϊ��ֹˮ��װ��G�м�ʯ�ҵ�Ӱ�죬�������װ��GǰӦ���и��ﴦ�������ԣ�װ��FӦ����Һ��������
��3��װ��B��ʢ�ŵ�����������Һ�����տ����еĶ�����̼������װ����ͨ�����ʱ��װ��B�е��������ƿ��ѿ����ж�����̼��ȥ�������˶�����̼��ʵ���Ӱ�죻
��4��װ��G�еļ�ʯ����ֱ���������ͨ����ʯ�����տ����е�ˮ�������̼��Ӱ��ʵ��Ľ��������һ��װ�м�ʯ�ҵ�װ��H���Ա������Ӱ�죻
��5���Ա�̽��ʵ�������ö�����̼������ʵ�ʿɵö�����̼���������������ʵ��̽���ж�����̼��������ƫ��IJ���ԭ��
��2��Ϊ��ֹˮ��װ��G�м�ʯ�ҵ�Ӱ�죬�������װ��GǰӦ���и��ﴦ�������ԣ�װ��FӦ����Һ��������
��3��װ��B��ʢ�ŵ�����������Һ�����տ����еĶ�����̼������װ����ͨ�����ʱ��װ��B�е��������ƿ��ѿ����ж�����̼��ȥ�������˶�����̼��ʵ���Ӱ�죻
��4��װ��G�еļ�ʯ����ֱ���������ͨ����ʯ�����տ����е�ˮ�������̼��Ӱ��ʵ��Ľ��������һ��װ�м�ʯ�ҵ�װ��H���Ա������Ӱ�죻
��5���Ա�̽��ʵ�������ö�����̼������ʵ�ʿɵö�����̼���������������ʵ��̽���ж�����̼��������ƫ��IJ���ԭ��
����⣺��1����ƽָ��ƫ�����̣�˵�����������������̣����ݡ��������롱��ԭ���ж����̵���Ʒ����������������������
�ʴ�Ϊ����Ʒ�أ������
��2��Ϊ��ֹˮ��Gװ���м�ʯ������ˮ��Ӱ��Զ�����̼�����IJⶨ����Ҫ��װ��Gǰ��Ũ�������������е�ˮ�����ԣ�װ��F��Ӧ����Ũ���
�ʴ�Ϊ��Ũ���
��3��������̼���������Ʒ�Ӧ����̼���ƺ�ˮ��װ��B�е�����������Һ���տ����еĶ�����̼�������˿����еĶ�����̼���ʵ���ж�����̼�����IJ���Ӱ�죻
�ʴ�Ϊ����ȥ�����еĶ�����̼��CO2+2NaOH=Na2CO3+H2O��
��4��װ��H�еļ�ʯ�ҿ����տ����еĶ�����̼��ˮ����ֹװ��G�����տ����ж�����̼��ˮ��Ӱ��ʵ��Բ���������̼�����IJⶨ��
�ʴ�Ϊ����ֹ�����еĶ�����̼��ˮ������װ��G�м�ʯ�����գ�
��5����ʵ��10.0g�ֲ�Ʒֻ�ܲ���0.44g CO2������̽�����������ƵõĶ�����̼����ȴΪ��0.48g��0.44g��˵��ʵ������л����ڲ���������̼�ķ�Ӧ��ʹ����������Ʒ��Ӧʱ�����������������̼�л��лӷ�����HCl���壬������ͨ��̼��������Һʱ������Ӧ�����Ķ�����̼ʹ�òⶨ���ƫ��
�ʴ�Ϊ��װ��D��Ũ����ӷ������Ȼ�����װ��E��NaHCO3��Ӧ����������̼��
�ʴ�Ϊ����Ʒ�أ������
��2��Ϊ��ֹˮ��Gװ���м�ʯ������ˮ��Ӱ��Զ�����̼�����IJⶨ����Ҫ��װ��Gǰ��Ũ�������������е�ˮ�����ԣ�װ��F��Ӧ����Ũ���
�ʴ�Ϊ��Ũ���
��3��������̼���������Ʒ�Ӧ����̼���ƺ�ˮ��װ��B�е�����������Һ���տ����еĶ�����̼�������˿����еĶ�����̼���ʵ���ж�����̼�����IJ���Ӱ�죻
�ʴ�Ϊ����ȥ�����еĶ�����̼��CO2+2NaOH=Na2CO3+H2O��
��4��װ��H�еļ�ʯ�ҿ����տ����еĶ�����̼��ˮ����ֹװ��G�����տ����ж�����̼��ˮ��Ӱ��ʵ��Բ���������̼�����IJⶨ��
�ʴ�Ϊ����ֹ�����еĶ�����̼��ˮ������װ��G�м�ʯ�����գ�
��5����ʵ��10.0g�ֲ�Ʒֻ�ܲ���0.44g CO2������̽�����������ƵõĶ�����̼����ȴΪ��0.48g��0.44g��˵��ʵ������л����ڲ���������̼�ķ�Ӧ��ʹ����������Ʒ��Ӧʱ�����������������̼�л��лӷ�����HCl���壬������ͨ��̼��������Һʱ������Ӧ�����Ķ�����̼ʹ�òⶨ���ƫ��
�ʴ�Ϊ��װ��D��Ũ����ӷ������Ȼ�����װ��E��NaHCO3��Ӧ����������̼��
��������ȡ��ѧ�仯�ⶨ�������ij��ɳɷֵĺ���ʱ���Է�Ӧ��ij���������IJ�����ֱ��Ӱ�����յ�̽�������

��ϰ��ϵ�д�
�����Ŀ