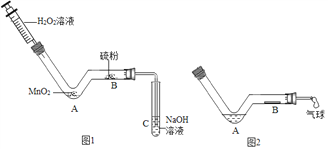
��Ŀ����
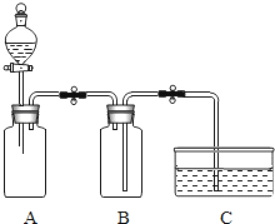
����Ŀ����һ�ձ���ʢ��22.3g Na2CO3��NaCl��ɵĹ������������ˮ�ܽ⣬�Ƴ���Һ���������μ�������������Ϊ10%��ϡ���ᣬ�ų��������������������ϡ�����������ϵ������ͼ��ʾ��
��1�����μ���73gϡ����ʱ���ų������������Ϊ g��
��2�����μ�ϡ������ͼ��B��ʱ���ձ�����Һ��������ǣ�д��ѧʽ�� ��
��3�����μ���73gϡ����ʱ����A��ʱ�����ձ���Ϊ��������Һ����ͨ��������������������ʵ�������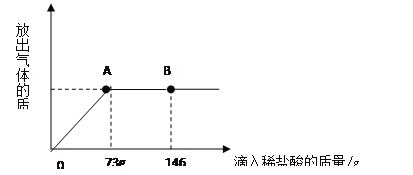
���𰸡���1��4.4���˿�2�֣�
��2��NaCl��HCl ���˿�2�֣�
��3������С��6�֣�
�⣺73g10%��ϡ�����к�HCl�������ǣ�
73g��10%=7.3g
��μӷ�Ӧ��̼���Ƶ�����Ϊx����Ӧ���ɵ��Ȼ��Ƶ�����Ϊy
Na2CO3+ 2HCl =" 2NaCl" + CO2�� + H2O
106 73 117
x 7.3g y
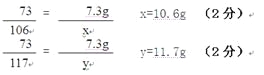
�ձ��ﲻ������Һ�����ʵ�����Ϊ�� 117.g + (22.3g��10.6g) =" 23.4g" ��2�֣�
�𣺣��ԣ�
����������������� ��1�����ݣ�3�������֪���������������Ϊ4.4 g
��2����ͼ��֪A�㴦��̼���ƺ����ᷴӦ��ȫ�������Ȼ��ƣ��μ����ᵽB����������� �ձ�����Һ��������ΪNaCl ��HCl
��3���⣺73g10%��ϡ�����к�HCl�������ǣ�73g��10%=7.3g
��μӷ�Ӧ��̼���Ƶ�����Ϊx����Ӧ���ɵ��Ȼ��Ƶ�����Ϊy���ɵĶ�����̼������Ϊz
Na2CO3+ 2HCl="==2NaCl" + CO2�� + H2O
106 73 117 44
x 7.3g y z
���x=10.6g y=11.7g z=4.4g
�ձ��ﲻ������Һ�����ʵ�����Ϊ�� 117.g + (22.3g-10.6g) = 23.4g

����Ŀ�� ijС��ͬѧ��������������ȼ������ʵ��ʱ����ʦ�����ڼ���ƿ�ײ�������NaOH��Һ�������ɵ�SO2����������������ͬѧ�ǵ�˼����SO2��NaOH�Ƿ�����Ӧ��
[��������]ͨ��״���£�1���ˮԼ���ܽ�40���SO2��
[ʵ�鷽��]ͬѧ�������SO2��������ƿ��Ѹ�ٵ���һ����NaOH��Һ��š��ƿ�ǣ�����������ƿ���������ΪSO2��NaOH�����˷�Ӧ��
[��˼��Ľ�]��ͬѧ��Ϊ����ʵ�鲻���Ͻ���������_____������ͬѧ����������������Ľ�������
����һ
ʵ����� | ʵ������ |
����������SO2��200mL������ƿ�зֱ�ע�� 10mLˮ��NaOH��Һ�����Աȡ� | ����ƿ�ӱ��ij̶���ȫ��ͬ |
��ͬѧ��Ϊ��������ƿ�ӱ��ij̶���ȫ��ͬ�������֤��SO2��NaOH�Ƿ����˷�Ӧ���Է�����������ƿ�ӱ��ij̶���ȫ��ͬ��ԭ����_____������Ϊ���ı�ע��ˮ��NaOH��Һ�������ɴﵽʵ��Ŀ�ģ�ע��Һ������������_____������ţ���
a��15mL b��8mL c��5mL d��3mL
������
ʵ����� | ʵ������ |
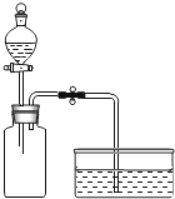
������ͼ��ʾװ�ý���ʵ�飺
����������SO2��300mL����ƿ�зֱ����6mLˮ��NaOH��Һ��һ��ʱ���ֹˮ�У��Աȡ� | ��������������ƿ�е�Һ�������ȫ��ͬ |
��ͬѧ��Ϊ��������Ҳ��֤��SO2��NaOH�Ƿ�����Ӧ������Ϊ��������Һ��������ȫ��ͬ��ԭ����_____��
��ͬѧ˼��������װ�ý����˸Ľ�����ﵽʵ��Ŀ�ġ��Ľ�װ����ͼ��ʾ������Ϊװ��B��������_____��