��Ŀ����
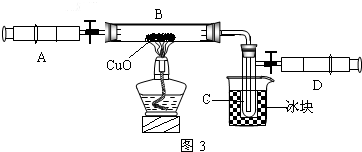
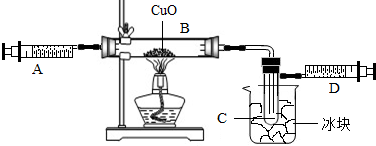
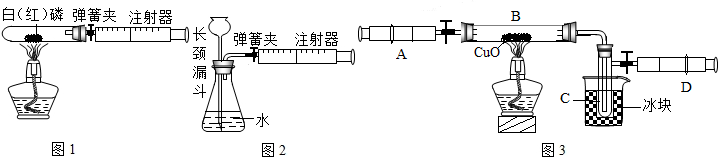
ijѧ��Ϊ�˲ⶨ������Ԫ���γɵ���̬������X����ɣ�������ͼ��ʾ��ʵ�飬������X��ע����A��������װ��CuO��Bװ�ã�ʹ֮��ȫ��Ӧ���õ����½����
��ʵ��ǰB�ܼ�ҩƷ������Ϊ21.3g��ʵ���Ϊ18.9g��
��C�����ռ��������ʵ���ɵõ�H2��O2����D���ռ���1.4gN2��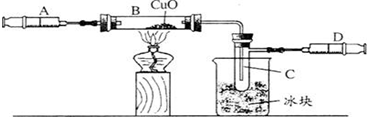
��ش��������⣺
��1��C���ռ�����Һ�壬������
��2����ʵ���п�����������
��3��X���ʵ���Է���������
��4��B�з�Ӧ�Ļ�ѧ����ʽ��
��ʵ��ǰB�ܼ�ҩƷ������Ϊ21.3g��ʵ���Ϊ18.9g��
��C�����ռ��������ʵ���ɵõ�H2��O2����D���ռ���1.4gN2��

��ش��������⣺
��1��C���ռ�����Һ�壬������
2.7
2.7
g����2����ʵ���п�����������
��ɫ������Ϊ��ɫ��C��������ɫҺ����֣�D���ڹ������ƶ�
��ɫ������Ϊ��ɫ��C��������ɫҺ����֣�D���ڹ������ƶ�
����3��X���ʵ���Է���������
17
17
����4��B�з�Ӧ�Ļ�ѧ����ʽ��
2NH3+3CuO
3Cu+3H2O+N2
| ||
2NH3+3CuO
3Cu+3H2O+N2
��
| ||
��������ʵ��ǰB�ܼ�ҩƷ������Ϊ21.3g��ʵ���Ϊ18.9g��C�����ռ��������ʵ���ɵõ�H2��O2����D���ռ�������N2�����ǿ��Եõ�X���е���������Ԫ�أ�Ȼ�����ˮ����Ԫ�غ͵����е�Ԫ�ص����������Ԫ�ص������Ƚ�������ȷ��X�Ļ�ѧʽ��
����⣺��1�������� B�ܷ�Ӧǰ����ٵ�����Ϊ����ͭ����Ԫ�ص�����������ͭ����Ԫ�ص����������ɵ�ˮ����Ԫ�ص�������ȣ���ˮ����Ԫ�ص�����=21.3g-18.9g=2.4g������ˮ������=2.4g��
��100%=2.7g��
��2������ͭ�백����Ӧ����ͭ��ˮ�͵�������������Ϊ��ɫ������Ϊ��ɫ��C��������ɫҺ����֣�D���ڹ������ƶ���
��3�����ǿɵ�X�к��е���������Ԫ�أ����е�Ԫ�غ���Ԫ����Ԫ�ص���������1.4g����2.7g-2.4g��=14��3���������ǿɵõ���������Ԫ�ص�ԭ�Ӹ�����Ϊ1��3�����Ի�ѧʽΪNH3����Է�������=14+1��3=17��
��4��B���ǰ���������ͭ��Ӧ����ͭ��������ˮ���仯ѧ����ʽΪ2NH3+3CuO
3CuʮN2+3H20��
�ʴ�Ϊ����1��2.7����2����ɫ������Ϊ��ɫ��C��������ɫҺ����֣�D���ڹ������ƶ���
��3��17����4��2NH3+3CuO
3Cu+3H2O+N2��
| 16 |
| 16+1��2 |
��2������ͭ�백����Ӧ����ͭ��ˮ�͵�������������Ϊ��ɫ������Ϊ��ɫ��C��������ɫҺ����֣�D���ڹ������ƶ���
��3�����ǿɵ�X�к��е���������Ԫ�أ����е�Ԫ�غ���Ԫ����Ԫ�ص���������1.4g����2.7g-2.4g��=14��3���������ǿɵõ���������Ԫ�ص�ԭ�Ӹ�����Ϊ1��3�����Ի�ѧʽΪNH3����Է�������=14+1��3=17��
��4��B���ǰ���������ͭ��Ӧ����ͭ��������ˮ���仯ѧ����ʽΪ2NH3+3CuO
| ||
�ʴ�Ϊ����1��2.7����2����ɫ������Ϊ��ɫ��C��������ɫҺ����֣�D���ڹ������ƶ���
��3��17����4��2NH3+3CuO
| ||
������װ��B������ͭ��Ϊͭ�����Լ��ٵ�����Ϊ����ͭ����Ԫ�ص�����������Ԫ�����백���е���Ԫ�ؽӺϳ�ˮ������ˮ����Ԫ�ص�������ˮ����Ԫ�ص�������ȣ��Ӷ����ˮ��������

��ϰ��ϵ�д�
 ÿ�α���ϵ�д�
ÿ�α���ϵ�д� ��ѧ����ϵ�д�
��ѧ����ϵ�д�
�����Ŀ