��Ŀ����
��2013?������һģ����Դ��������������������ᷢչ������أ�
��1������������δ��������������Դ��������ȼ�ϳ�ȼ��ʱ�����϶�������⣬��һ���ŵ���
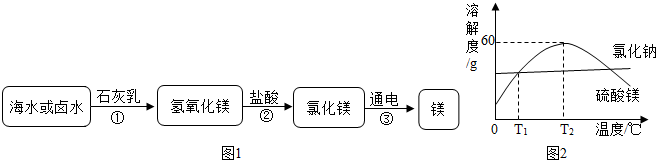
��2��Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ��Ϊ������Ⱦ�����ú�������ʣ��ɽ���ת��Ϊ��ȼ�����壬�˹��̿���Ϊ��̼��ˮ�ķ�Ӧ������ʾ��ͼ��ͼ��ʾ��

����˵����ȷ����
�ٷ�Ӧǰ����ӵ�����û�иı�
�ڷ�Ӧǰ������Ԫ�صĻ��ϼ۶�û�б仯
�۲μӷ�Ӧ��A��B�����ʵ�����������1��3
�ܸ÷�Ӧ���û���Ӧ
��3��Ϊ�������������ŷţ����ǻ���Ѱ�Ҳ���̼Ԫ�ص�ȼ�ϣ����о�����NH3��ȫȼ������ˮ�����Ϳ����к����������壬����û����Ⱦ�����ͷŴ�����������һ��Ӧ��ǰ����
��NH3�е�Ԫ�غ���Ԫ�ص�������Ϊ
��д��NH3ȼ�յĻ�ѧ����ʽ
��1������������δ��������������Դ��������ȼ�ϳ�ȼ��ʱ�����϶�������⣬��һ���ŵ���
ȼ�ղ��ﲻ��Ⱦ����
ȼ�ղ��ﲻ��Ⱦ����
����2��Ŀǰ�������Ի�ʯȼ��Ϊ��Ҫ��Դ��Ϊ������Ⱦ�����ú�������ʣ��ɽ���ת��Ϊ��ȼ�����壬�˹��̿���Ϊ��̼��ˮ�ķ�Ӧ������ʾ��ͼ��ͼ��ʾ��

����˵����ȷ����
��
��
������ţ����ٷ�Ӧǰ����ӵ�����û�иı�
�ڷ�Ӧǰ������Ԫ�صĻ��ϼ۶�û�б仯
�۲μӷ�Ӧ��A��B�����ʵ�����������1��3
�ܸ÷�Ӧ���û���Ӧ
��3��Ϊ�������������ŷţ����ǻ���Ѱ�Ҳ���̼Ԫ�ص�ȼ�ϣ����о�����NH3��ȫȼ������ˮ�����Ϳ����к����������壬����û����Ⱦ�����ͷŴ�����������һ��Ӧ��ǰ����
��NH3�е�Ԫ�غ���Ԫ�ص�������Ϊ
14��3
14��3
����д��NH3ȼ�յĻ�ѧ����ʽ
4NH3+3O2
6H2O+2N2
| ||
4NH3+3O2
6H2O+2N2
��
| ||
��������1������������ֵ�ߡ�ȼ�ղ�����ˮ������Ⱦ��������һ���������Դ��
��2�����ݷ�Ӧ��������ʾ��ͼ�������ж���ط�������⣻
��3���������ʵĻ�ѧʽ���Լ������Ԫ�ص������ȣ����ݷ�Ӧ��������Ӧ�������������غ㶨�ɿ�����д��ѧ����ʽ��
��2�����ݷ�Ӧ��������ʾ��ͼ�������ж���ط�������⣻
��3���������ʵĻ�ѧʽ���Լ������Ԫ�ص������ȣ����ݷ�Ӧ��������Ӧ�������������غ㶨�ɿ�����д��ѧ����ʽ��
����⣺��1������ȼ��������ˮ������Ⱦ������
���ȼ�ղ��ﲻ��Ⱦ������
��2��̼��ˮ�����ڸ��������·�Ӧ������һ����̼����������ѧ����ʽΪ��C+H2O
CO+H2��
��Ӧǰ����ӵ�������˸ı䣻
��Ӧǰ��̼Ԫ�ء���Ԫ�صĻ��ϼ۷����˸ı䣻
�۲μӷ�Ӧ��A��B�����ʵ�����������1��1��
�ܸ÷�Ӧ�У���Ӧ��������ﶼ��һ�ֵ��ʺ�һ�ֻ�������û���Ӧ��
����ܣ�
��3����NH3�е�Ԫ�غ���Ԫ�ص�������Ϊ����14��1������1��3��=14��3��
���14��3��
�ڿ����к������������ǵ���������ȼ��������ˮ�͵�������Ӧ�Ļ�ѧ����ʽΪ��4NH3+3O2
6H2O+2N2��
���4NH3+3O2
6H2O+2N2��
���ȼ�ղ��ﲻ��Ⱦ������
��2��̼��ˮ�����ڸ��������·�Ӧ������һ����̼����������ѧ����ʽΪ��C+H2O
| ||
��Ӧǰ����ӵ�������˸ı䣻
��Ӧǰ��̼Ԫ�ء���Ԫ�صĻ��ϼ۷����˸ı䣻
�۲μӷ�Ӧ��A��B�����ʵ�����������1��1��
�ܸ÷�Ӧ�У���Ӧ��������ﶼ��һ�ֵ��ʺ�һ�ֻ�������û���Ӧ��
����ܣ�
��3����NH3�е�Ԫ�غ���Ԫ�ص�������Ϊ����14��1������1��3��=14��3��
���14��3��
�ڿ����к������������ǵ���������ȼ��������ˮ�͵�������Ӧ�Ļ�ѧ����ʽΪ��4NH3+3O2
| ||
���4NH3+3O2
| ||
�������ڻ�ѧ��Ӧ����ѭ�����غ㶨�ɣ�����Ӧǰ��Ԫ�ص�����䣬ԭ�ӵ����ࡢ�������䣮

��ϰ��ϵ�д�
�����Ŀ