��Ŀ����
ˮ����Һ�����ǵ���������������ء�
��1���ҹ����Ƴ���Ư�۸���Ч������ˮ��������ClO2���������ȡClO2��Ӧ�Ļ�ѧ����ʽ��Cl2+ 2NaClO2= 2ClO2+________��
��2���·���մ�е����ۿ�������ϴȥ������Ϊ������____����ܽ⡱���黯�������ۡ�
��3����0��ʱ���������ܽ��Ϊ0.024�ĺ�����___________________________________��
��4��M���ʵ��ܽ�����¶ȵ����߶�����80gM���ʼ���50gˮ�У�����ܽ⣬�����Һ���������¶ȵı仯������ͼ��ʾ��
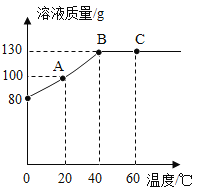
��A��ʱ��Һ��ʣ����������Ϊ________g��
��A��B��C��������Ӧ����Һ�����ڱ�����Һ����____(����ĸ)��
����40��ʱ��M���ʵ��ܽ��Ϊ_______g��
���������ܼ�����������,��C���Ӧ����Һ���M�ľ��壬���Բ��õķ�����_________��
��5��ʵ������68g��������Ϊ5%�Ĺ���������Һ��ȡ������������������ȫ�ֽ⣬���ɲ��������������Ƕ��٣���д��������̣�___
 ������ĩ��ϰ��ѵ��ϵ�д�
������ĩ��ϰ��ѵ��ϵ�д� С��ʿ��ĩ����100��ϵ�д�
С��ʿ��ĩ����100��ϵ�д�Ϊ��ȥ���������е����ʣ����в��������ܴﵽĿ�ĵ���
ѡ�� | ���ʣ����ʣ� | �������� |
A | CO2���壨H2O�� | ������ͨ��ʢ���������ĸ���� |
B | CuO���壨CuSO4�� | �������ϡ���Ტ���� |
C | FeCl2��Һ��CuCl2�� | ������������ۣ������� |
D | Na2SO4��Һ��Na2CO3�� | ��ϡ������ǡ�ò��ٲ�������Ϊֹ |
A.A B.B C.C D.D