��Ŀ����
����Ŀ����11�����ں�ۡ��ۺͷ���֮�佨����ϵ�ǻ�ѧѧ�Ƶ��ص㡣
(1����Ҫ����дָ���Ļ�ѧ���Ż���ŵ�����
��ѧ���� | 2Cu | 3CO32- | ||
���ŵ����� | 2�������� | �������� |
��2��ͼ2��ˮ�����ס������ֱ仯���۽ṹģ�͡�
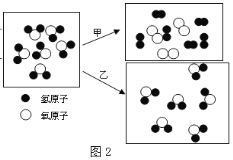
���ס������ֱ仯�����������仯����__ _ _��������_ _��
���仯�����ֱ���ʽΪ ��
�����̼������ܹ�˵��ˮ�Ļ�ѧʽΪ��H2O���������� ��
��3����Ԫ�ض����彡������Ҫ�����������ij���Ԫ�أ�ÿ�ձ��������㹻���ĸ���Ŀǰ�г��ϵIJ���ҩ���ܶ࣬��ͼ��ij��Ʒ�ƵIJ���ҩƷ�IJ���˵��������ش��������⣺

��CaCO3��̼����Ԫ������������ ��
��CaCO3�и�Ԫ�ص���������Ϊ ��
��������������ã�ÿ�������Ԫ�ص�����Ϊ g��
���𰸡�(1��2��ͭԭ�� 2O2 3��̼������� NaOH
��2������ ���ӵ�����û�з����ı�(��û��������������
��ˮ![]() ����+ ����
����+ ����
�� 6��ˮ���ӷֽ��õ�6������Ӻ�3�������ӣ�(��ÿ������Ӻ�ÿ���������и���2��ԭ�ӡ���
��3����1��4 ��40% ��0��3
��������
���������(1�� 2Cu��ʾ����2��ͭԭ���������ӵĻ�ѧ����Ϊ��O2������������Ӧ�ñ�ʾΪ��2O2 ��3CO32- ��ʾ������Ϊ��3��̼����������������ƵĻ�ѧ����Ϊ��NaOH��
��2��������ԭͼ��ȣ����Ӹı��ˣ����ڻ�ѧ�仯������ԭͼ��ȣ�����û�иı䣬�ı��֪ʶ����֮��ļ�϶�����������仯��
��������ʾ��ͼ��֪��ˮ���ӱ���������Ӻ�����ӣ��ʱ仯�����ֱ���ʽΪ��ˮ![]() ����+ ������
����+ ������
�����ݼ���ʾ��ͼ��֪��6��ˮ���ӷֽ��õ�6������Ӻ�3�����������۲��֪��ÿ��ˮ��������������ԭ�Ӻ�һ����ԭ�ӣ���֪ˮ�Ļ�ѧʽΪH2O��
��3����CaCO3��̼����Ԫ������������12����16*3��=1��4
��CaCO3�и�Ԫ�ص���������Ϊ��![]() ��
��
��ÿ�������Ԫ�ص�����Ϊ��0��25g*3*40%=0��3��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ����ȥ���������е����ʣ�������Ϊ���ʣ�����ѡ�õ��Լ���������������ȷ��һ����
ѡ�� | ���ᴿ������ | ѡ�õ��Լ� | �����ķ��� |
A | CaO��CaCO3�� | ˮ | �ܽ⡢���ˡ��ᾧ |
B | CuSO4��H2SO4�� | ����������Һ | ���� |
C | Cu��CuO�� | ϡ���� | �ܽ⡢���ˡ�ϴ�ӡ����� |
D | CO2��CO�� | ���� | ��ȼ |