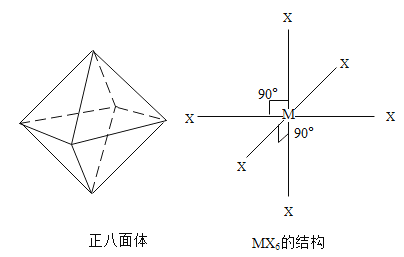
��Ŀ����
����Ŀ�������±������ֻ��ʵ���Ϣ���ش��й����⡣
���� | ���� | ����� | ̼����� |
��ѧʽ | CO��NH2��2 | NH4NO3 | NH4HCO3 |
�г��۸� | 1080Ԫ/�� | 810Ԫ/�� | 330Ԫ/�� |
��1��̼����淋���Է�������Ϊ_____��
��2��������е�Ԫ�ص���������Ϊ_____��
��3��������������̼Ԫ������Ԫ�ص�������Ϊ_____��
��4���ֱ���10000Ԫ�ɹ����ء�����李�̼��������ֻ��ʣ������õĻ����е�Ԫ�ص������ֱ�Ϊx��y��z����x��y��z֮��Ĺ�ϵ��x_____y_____z���ã�������ʾ����
���𰸡�79 35% 3��1 �� ��
��������
��1��������Է��ӵ�����Ϊ��ɷ��ӵĸ�ԭ�ӵ����ԭ������֮�ͣ��ɵ�̼����淋���Է�������Ϊ��14+5+12+16��3��79��
��2��������е�Ԫ�ص���������Ϊ=![]() ��
��
��3��������������̼Ԫ������Ԫ�ص�������Ϊ12����1��4����3��1��
��4��1��Ԫ����Ļ���������Ԫ�ص������ֱ�Ϊ��
CO��NH2��2�е�Ԫ�ص�������x��=![]() ��
��
NH4NO3�е�Ԫ�ص�������y��=![]() ��
��
NH4HCO3 �е�Ԫ�ص�������z��=![]() ��
��
����x��y��z��

 ��ʦ������Ԫ��ĩ���100��ϵ�д�
��ʦ������Ԫ��ĩ���100��ϵ�д� ��У������Ԫͬ��ѵ��������ϵ�д�
��У������Ԫͬ��ѵ��������ϵ�д�����Ŀ��ijУͬѧ�ǿ�չ������ѧ���ʵ�ʵ��̽�����
��1��ijͬѧ��Ʋ�ͬʵ�鷽������֤����ͭ���ֽ������
ʵ�鲽�� | ʵ������ | ʵ����� |
�ٷֱ�ȡ��������Ƭ��ͭƬ�����Թ��У����������ϡ���� | ________ | ����ͭ���ǿ |
��________ | ________ |
��2��ijС����þ��ϡ���ᷴӦʵ��ʱ�����ָ÷�Ӧ�ܾ��ң����۲쵽�Թ��ڲ���������������������������������̽��:��Ӧ�����У��������� ������ԭ����________________________��д��þ��ϡ���ᷴӦ�Ļ�ѧ����ʽ____________________����ȼ�ŵ�ľ�������Թܿڣ�������������,���Թ�û�б�ը��ԭ����____________________��
[�������]ʵ������У����ֻ��������ȼ�յı�������û�п������棬С���������ʵ������������ȼ�ղ���������ɫ������?
[��������]ˮ���Ĵ��ڣ�������ȼ�ջ���Ĵ����к����Ե��������ã���ˮ����Ũ��Խ�ߣ����洫�����ٶ�Խ����
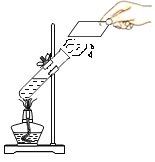
[���ʵ��]����ʦָ����ͬѧ�ǽ�ʵ������˸Ľ�����ͼ��ʾ��
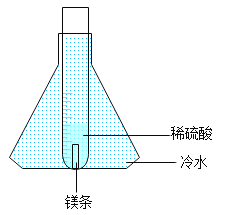
[ʵ�����]ȡ0.3 gþ������װ��5.0 gϡ������Թ��У����Թܷ���ʢ����ˮ����ƿ�У���Ӧ��ʼ����ȼ�ŵ�ľ����ȼ�Թܿڵ��������۲쵽��������ȼ�գ�������ֵ���ɫ��
[�����뷴˼]����ƿ����ˮ��������__________��
����ʵ����ȴ�ָ����µĹ����У�ͬѧ�ǻ�����þ����ʧһ��ʱ����Թ��ڲ������˰�ɫ���塣�Դ�����������������Ľ�����________��������ʱ��Һ�����ʵ���������Ϊ________���г�����ʽ���ɣ���������������þ���ܽ��Ϊ33.5 g��
����Ŀ������ʵ�鷽����ʵ��������Ӧ���ǣ�������
ѡ�� | �� | �� | �� | �� |
���� |
|
|
|
|
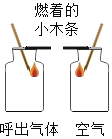
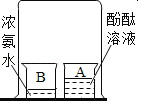
���� | װ�к�������ļ���ƿ�е�Сľ��Ϩ��װ�п����ļ���ƿ�е�Сľ�������Ա仯 | A�ձ��еķ�̪��Һ��죬B�ձ��е�Ũ��ˮ�����Ա仯 | ����Ƭ����Сˮ����� | ��������İ��̱�����ȼ��ʹ��������ȼ�� |
���� | ���������еĶ�����̼�����ȿ����еĶ� | �������ڲ����˶�������̪���Ӳ��˶� | Һ̬ˮ����̬ˮ�����ת�� | ���̵ijɷֲ��Ƕ�����̼ |
A.�٢�B.�ڢ�C.�ۢ�D.�٢�
����Ŀ��ij������ȤС���ͬѧ�Զ�����̼����ȡ�����ʽ������̽����
��ʵ��عˣ���1��ʵ�����ô���ʯ��ϡ������ȡ������̼�Ļ�ѧ����ʽΪ_______________��
��2����ͼ������ʵ������ȡCO2����װ�õ���__________������ĸ����
��ʵ��̽����CO2��NaOH��Һ��Ӧ
���������ϣ�
��1������CO2ͨ��NaOH��Һ������������Ӧ��
��һ����____________________________���û�ѧ����ʽ��ʾ��
�ڶ�����Na2CO3+H2O+CO2=2NaHCO3
��2��Na2CO3��NaHCO3�����ܽ�ȱ�
�¶�/�� | 0 | 15 | 20 | 30 | 40 | 50 | 60 |
NaHCO3/g | 6.9 | 8.72 | 9.6 | 11.1 | 12.7 | 14.45 | 16.4 |
Na2CO3/g | 7.1 | 13.25 | 21.8 | 39.7 | 48.8 | 47.3 | 46.4 |
��ʵ����ƣ�������Ϊ15��ʱ����10gNaOH������ȫ�ܽ���80gˮ�У���������ͨ��CO2��ͬʱ�ô������ⶨ��Һ��pH�仯�������ͼ1��ʾ������ظ�ʵ�飬����ʵ��������һ�¡�
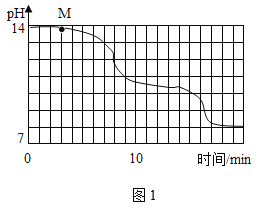
��1��ͨ��ͼ�������NaHCO3��Һ��_____������ԡ��������ԡ����ԡ�����
��2��Ϊ��ȷ��M����Һ�ijɷ֣�ͬѧ�ǽ�������ʵ�飺
ʵ�鲽�� | ʵ������ | ����ʵ����� |
��ȡ�����μӹ�����BaCl2��Һ | ������ɫ���� | ����Na2CO3��NaOH |
���ڢٷ�Ӧ����ϲ���Һ�еμ�____ | _____ |
��3��ʵ������У�ͬѧ�Ƿ���18min����Һ��pH�������ٱ仯�����ʱ��Һ�пɹ۲쵽��������______________________________��
����չ���죩ijͬѧ������������̼ԭ������̼�����ƺ�̼������Һ�У��ֱ���μ�����ͬŨ�ȵ�ϡ���ᣬ�����������������CO2������֮���ϵ��ͼ2��ͼ3��ʾ��(����CO2��ˮ�е��ܽ�)

��1��ͼ2��A����Һ�е�������_________��д��ѧʽ ����ͼ3�д�B�㿪ʼ�ı仯��ϵ��ͼ2��ȫ�غ�,ͼ3��B����Һ�е�������__________��д��ѧʽ����
��2��д��OB�η�����Ӧ�Ļ�ѧ����ʽ______________________________��