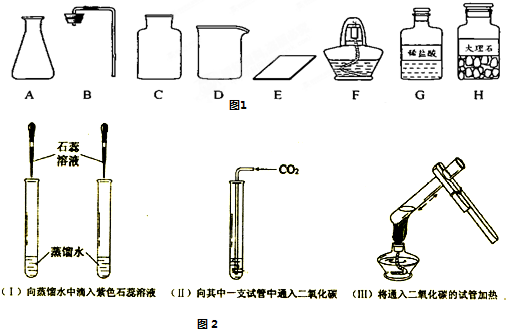
��Ŀ����
�γ���2013���п���ѧʵ�鿼���У�����������ȡ���ڶ�����̼����ȡ��������50g 5%��NaCl��Һ������������ǩ,��ѧ����ǩȷ��һ��������п��顣
��1����ͬѧ��ǩ������ʵ���ң����ֱ���ʵ��������Ҫ��������������ҩƷ��
�� ��ͼ������D�������� ��F�������� ��
��ͼ������D�������� ��F�������� ��
�ڼ�ͬѧ�鵽�Ŀ�ǩӦ���� ������ĸ���ţ���
A����������ȡ B�� ������̼����ȡ
����ȡ������ķ�Ӧԭ��Ϊ ���û�ѧ����ʽ��ʾ������ȡһƿ�����壬Ӧѡ��
�������� ������ĸ���ţ���
�ܼ�ͬѧʵ�����Ҫ����ʾ�����£������������������
���װ�á���������ԡ� ���ռ����塣
�������������巢��װ�û�������ȡ�������壬��д������һ�ַ�Ӧ�Ļ�ѧ����ʽ ��
��2����ͬѧ����ɡ�����50g 5%��NaCl��Һ��ʵ������У�ͨ�����㣬�����NaCl g����ȡˮԼ mL���ܽ�NaClʱ�õ��IJ������������� �� ��
��1�����ձ����ƾ���
��B�� ��CaCO3 +2 HCl == CaCl2 +H2O +CO2����A B C E
���ȼӴ���ʯ���ϡ�����2H2O2����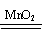 �� 2H2O + O2����
�� 2H2O + O2����
��2��2.5��47.5�����裬�ӿ��ܽ��ٶ�