��Ŀ����
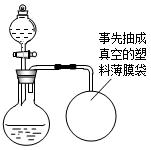
ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ����ʲ������ᷴӦ��Ҳ������Ԫ�أ�����ǩ������ͼ��Ϊ�˲ⶨ�京��������С�飮���õ�����ƽ�ⶨ���й�ʵ���������±���

| ������Ŀ | ����/g |
| ����̼�����Ʒ | 10.00 |
| ��ƿ | 100.00 |
| ��ƿ+ϡ���ᣨ������ | 139.20 |
| ��ƿ+ϡ����+���������Ӧ��ʼ15�룩 | 145.60 |
| ��ƿ+ϡ����+���������Ӧ��ʼ35�룩 | 145.24 |
| ��ƿ+ϡ����+���������Ӧ��ʼ55�룩 | 145.20 |
��1����Ӧ�����Ķ�����̼�����������
��2����Ʒ��̼��Ƶ�����������
��3��ͨ������˵����ǩ��ʾ�ĺ������Ƿ���ȷ��
�⣺��1�����������غ㶨�ɣ�mCO2=10.00+139.20-145.20=4g���ʴ�Ϊ��4g
��2���⣺�����������CaCO3������ΪX
CaCO3+2HCl�TCaCl2+H2O+CO2�� ��1�֣�
100 44
X 4g

X��9.1g
��Ʒ��CaCO3%Ϊ=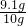 ��100%�T91%
��100%�T91%
����Ʒ��̼��Ƶ�����������91%��
�ʴ�Ϊ��91%
��3��10g̼�����Ʒ�и�Ԫ������=10g��91%��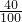 ��100%=3.64g
��100%=3.64g
��Ʒ�и�Ԫ�ص���������= ��100%=36.4%��35%
��100%=36.4%��35%
���ǩ��ʾ�ĺ�������ȷ
�𣺱�ǩ��ʾ�ĺ�������ȷ��
�ʴ�Ϊ����ǩ��ʾ�ĺ�������ȷ
��������1�������غ㶨�ɣ����ٵ������Ƿų������������
��2�����ݻ�ѧ����ʽ����IJ����У�һ�衢��д�����ҡ����С�������飮
��3��ͨ������ó����������ٽ��бȽϣ�
�����������㿼���˸��ݻ�ѧ����ʽ�ļ�������ʵ������������Ǹ��������غ㶨�������壬��ס��������IJ������壮����ʱҪע�⣺��ѧ����ʽҪд��ȷ��ʼ�ղ�Ҫ���������غ㶨���ڼ������е�Ӧ�ã���������Ҫ�����ڼ������У�
��2���⣺�����������CaCO3������ΪX
CaCO3+2HCl�TCaCl2+H2O+CO2�� ��1�֣�
100 44
X 4g

X��9.1g
��Ʒ��CaCO3%Ϊ=
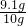 ��100%�T91%
��100%�T91% ����Ʒ��̼��Ƶ�����������91%��
�ʴ�Ϊ��91%
��3��10g̼�����Ʒ�и�Ԫ������=10g��91%��
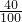 ��100%=3.64g
��100%=3.64g ��Ʒ�и�Ԫ�ص���������=
 ��100%=36.4%��35%
��100%=36.4%��35% ���ǩ��ʾ�ĺ�������ȷ
�𣺱�ǩ��ʾ�ĺ�������ȷ��
�ʴ�Ϊ����ǩ��ʾ�ĺ�������ȷ
��������1�������غ㶨�ɣ����ٵ������Ƿų������������
��2�����ݻ�ѧ����ʽ����IJ����У�һ�衢��д�����ҡ����С�������飮
��3��ͨ������ó����������ٽ��бȽϣ�
�����������㿼���˸��ݻ�ѧ����ʽ�ļ�������ʵ������������Ǹ��������غ㶨�������壬��ס��������IJ������壮����ʱҪע�⣺��ѧ����ʽҪд��ȷ��ʼ�ղ�Ҫ���������غ㶨���ڼ������е�Ӧ�ã���������Ҫ�����ڼ������У�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
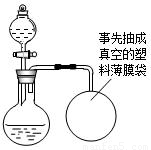
 ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ������в�����Ԫ�أ�����ǩ��ͼ��Ϊ�˲ⶨ�京��������С�������ͼװ�ý���ʵ�飬ʵ�鲽�����£�
ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ������в�����Ԫ�أ�����ǩ��ͼ��Ϊ�˲ⶨ�京��������С�������ͼװ�ý���ʵ�飬ʵ�鲽�����£�| XX�� ����̼��� ���������ƣ�35% ������25kg XX��������Ʒ�ټ��װ�õ������ԣ� ����Բ����ƿ�м�����Ʒ5.0g�� ��ͨ����Һ©����Բ����ƿ�л�������112mL������ϡ���ᣮ �ܴ���Ӧ�ָ������º���ռ�������������Ϊ1008mL�� ��֪�ڴ�ʵ�������£�ÿ112mL������̼���������Ϊ0.22g������ ��1����Ӧ�����Ķ�����̼����������� ��2����Ʒ��̼��Ƶ����������� ��3��ͨ������˵����ǩ��ʾ�ĺ������Ƿ���ȷ��
|
| XX�� ����̼��� ���������ƣ�35% ������25kg XX��������Ʒ |
����Բ����ƿ�м�����Ʒ5.0g��
��ͨ����Һ©����Բ����ƿ�л�������112mL������ϡ���ᣮ
�ܴ���Ӧ�ָ������º���ռ�������������Ϊ1008mL��
��֪�ڴ�ʵ�������£�ÿ112mL������̼���������Ϊ0.22g������
��1����Ӧ�����Ķ�����̼�����������
��2����Ʒ��̼��Ƶ�����������
��3��ͨ������˵����ǩ��ʾ�ĺ������Ƿ���ȷ��
ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ����ʲ������ᷴӦ��Ҳ������Ԫ�أ�����ǩ������ͼ��Ϊ�˲ⶨ�京��������С�飮���õ�����ƽ�ⶨ���й�ʵ���������±���

����
��1����Ӧ�����Ķ�����̼�����������
��2����Ʒ��̼��Ƶ�����������
��3��ͨ������˵����ǩ��ʾ�ĺ������Ƿ���ȷ��

| ������Ŀ | ����/g |
| ����̼�����Ʒ | 10.00 |
| ��ƿ | 100.00 |
| ��ƿ+ϡ���ᣨ������ | 139.20 |
| ��ƿ+ϡ����+���������Ӧ��ʼ15�룩 | 145.60 |
| ��ƿ+ϡ����+���������Ӧ��ʼ35�룩 | 145.24 |
| ��ƿ+ϡ����+���������Ӧ��ʼ55�룩 | 145.20 |
��1����Ӧ�����Ķ�����̼�����������
��2����Ʒ��̼��Ƶ�����������
��3��ͨ������˵����ǩ��ʾ�ĺ������Ƿ���ȷ��
ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ������в�����Ԫ�أ�����ǩ��ͼ��Ϊ�˲ⶨ�京��������С�������ͼװ�ý���ʵ�飬ʵ�鲽�����£�
�ټ��װ�õ������ԣ�
����Բ����ƿ�м�����Ʒ5.0g��
��ͨ����Һ©����Բ����ƿ�л�������112mL������ϡ���ᣮ
�ܴ���Ӧ�ָ������º���ռ�������������Ϊ1008mL��
��֪�ڴ�ʵ�������£�ÿ112mL������̼���������Ϊ0.22g������
��1����Ӧ�����Ķ�����̼�����������
��2����Ʒ��̼��Ƶ�����������
��3��ͨ������˵����ǩ��ʾ�ĺ������Ƿ���ȷ��
| XX�� ����̼��� ���������ƣ�35% ������25kg XX��������Ʒ |
����Բ����ƿ�м�����Ʒ5.0g��
��ͨ����Һ©����Բ����ƿ�л�������112mL������ϡ���ᣮ
�ܴ���Ӧ�ָ������º���ռ�������������Ϊ1008mL��
��֪�ڴ�ʵ�������£�ÿ112mL������̼���������Ϊ0.22g������
��1����Ӧ�����Ķ�����̼�����������
��2����Ʒ��̼��Ƶ�����������
��3��ͨ������˵����ǩ��ʾ�ĺ������Ƿ���ȷ��

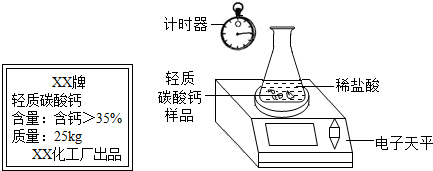
 ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ������в�����Ԫ�أ�����ǩ��ͼ��Ϊ�˲ⶨ�京��������С�������ͼװ�ý���ʵ�飬ʵ�鲽�����£�
ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ������в�����Ԫ�أ�����ǩ��ͼ��Ϊ�˲ⶨ�京��������С�������ͼװ�ý���ʵ�飬ʵ�鲽�����£�