��Ŀ����
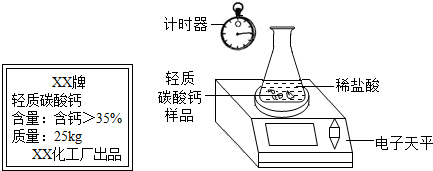
 ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ������в�����Ԫ�أ�����ǩ��ͼ��Ϊ�˲ⶨ�京��������С�������ͼװ�ý���ʵ�飬ʵ�鲽�����£�
ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ������в�����Ԫ�أ�����ǩ��ͼ��Ϊ�˲ⶨ�京��������С�������ͼװ�ý���ʵ�飬ʵ�鲽�����£�
| XX�� ����̼��� ���������ƣ�35% ������25kg XX��������Ʒ |
����Բ����ƿ�м�����Ʒ5.0g��
��ͨ����Һ©����Բ����ƿ�л�������112mL������ϡ���ᣮ
�ܴ���Ӧ�ָ������º���ռ�������������Ϊ1008mL��
��֪�ڴ�ʵ�������£�ÿ112mL������̼���������Ϊ0.22g������
��1����Ӧ�����Ķ�����̼�����������
��2����Ʒ��̼��Ƶ�����������
��3��ͨ������˵����ǩ��ʾ�ĺ������Ƿ���ȷ��
�⣺��1����Ӧ����������̼���������=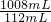 ��0.22g=1.98g
��0.22g=1.98g
��2����̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 1.98g
100��44=x��1.98g ��֮�� x=4.5g
��Ʒ��̼��Ƶ���������= ��100%=90%
��100%=90%
����Ʒ��̼��Ƶ���������Ϊ90%��
��3��4.5g̼����и�Ԫ������=4.5g��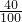 ��100%=1.8g
��100%=1.8g
��Ʒ�и�Ԫ�ص���������= ��100%=36%��35%
��100%=36%��35%
���ǩ��ʾ�ĺ�������ȷ
�𣺱�ǩ��ʾ�ĺ�������ȷ��
��������1�����ݶ�����̼����������Ĺ�ϵ���������ɶ�����̼��������ת����������
��2���ɶ�����̼�����������÷�Ӧ�Ļ�ѧ����ʽ������Ʒ��̼��Ƶ������������Ʒ��̼��Ƶ�����������
��3����̼����и�Ԫ�����������Ʒ�и�Ԫ�����������������ǩ�иƺ����Աȣ��жϱ�ǩ�Ƿ���ȷ��
��������ѧ�����������������ϣ����ֻ�ѧ�����������е�Ӧ�ã�����ѧϰ��ѧ����Ȥ��
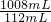 ��0.22g=1.98g
��0.22g=1.98g��2����̼��Ƶ�����Ϊx
CaCO3+2HCl=CaCl2+H2O+CO2��
100 44
x 1.98g
100��44=x��1.98g ��֮�� x=4.5g
��Ʒ��̼��Ƶ���������=
 ��100%=90%
��100%=90%����Ʒ��̼��Ƶ���������Ϊ90%��
��3��4.5g̼����и�Ԫ������=4.5g��
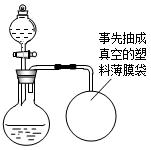
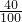 ��100%=1.8g
��100%=1.8g��Ʒ�и�Ԫ�ص���������=
 ��100%=36%��35%
��100%=36%��35%���ǩ��ʾ�ĺ�������ȷ
�𣺱�ǩ��ʾ�ĺ�������ȷ��
��������1�����ݶ�����̼����������Ĺ�ϵ���������ɶ�����̼��������ת����������
��2���ɶ�����̼�����������÷�Ӧ�Ļ�ѧ����ʽ������Ʒ��̼��Ƶ������������Ʒ��̼��Ƶ�����������
��3����̼����и�Ԫ�����������Ʒ�и�Ԫ�����������������ǩ�иƺ����Աȣ��жϱ�ǩ�Ƿ���ȷ��
��������ѧ�����������������ϣ����ֻ�ѧ�����������е�Ӧ�ã�����ѧϰ��ѧ����Ȥ��

��ϰ��ϵ�д�
�����Ŀ
 ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ������в�����Ԫ�أ�����ǩ��ͼ��Ϊ�˲ⶨ�京��������С�������ͼװ�ý���ʵ�飬ʵ�鲽�����£�
ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ������в�����Ԫ�أ�����ǩ��ͼ��Ϊ�˲ⶨ�京��������С�������ͼװ�ý���ʵ�飬ʵ�鲽�����£�| XX�� ����̼��� ���������ƣ�35% ������25kg XX��������Ʒ�ټ��װ�õ������ԣ� ����Բ����ƿ�м�����Ʒ5.0g�� ��ͨ����Һ©����Բ����ƿ�л�������112mL������ϡ���ᣮ �ܴ���Ӧ�ָ������º���ռ�������������Ϊ1008mL�� ��֪�ڴ�ʵ�������£�ÿ112mL������̼���������Ϊ0.22g������ ��1����Ӧ�����Ķ�����̼����������� ��2����Ʒ��̼��Ƶ����������� ��3��ͨ������˵����ǩ��ʾ�ĺ������Ƿ���ȷ��
ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ����ʲ������ᷴӦ��Ҳ������Ԫ�أ�����ǩ������ͼ��Ϊ�˲ⶨ�京��������С�飮���õ�����ƽ�ⶨ���й�ʵ���������±���

��1����Ӧ�����Ķ�����̼����������� ��2����Ʒ��̼��Ƶ����������� ��3��ͨ������˵����ǩ��ʾ�ĺ������Ƿ���ȷ��
ij��ȤС�鹺������ʳ�þ���ֲ������̼��ƣ������в�����Ԫ�أ�����ǩ��ͼ��Ϊ�˲ⶨ�京��������С�������ͼװ�ý���ʵ�飬ʵ�鲽�����£�
����Բ����ƿ�м�����Ʒ5.0g�� ��ͨ����Һ©����Բ����ƿ�л�������112mL������ϡ���ᣮ �ܴ���Ӧ�ָ������º���ռ�������������Ϊ1008mL�� ��֪�ڴ�ʵ�������£�ÿ112mL������̼���������Ϊ0.22g������ ��1����Ӧ�����Ķ�����̼����������� ��2����Ʒ��̼��Ƶ����������� ��3��ͨ������˵����ǩ��ʾ�ĺ������Ƿ���ȷ�� 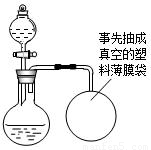 |