��Ŀ����
�����ʵ����Լ����ɴ�ʯ��ʯ����Ҫ�ɷ���CaCO3���л�øߴ���CaCO3�����������£�
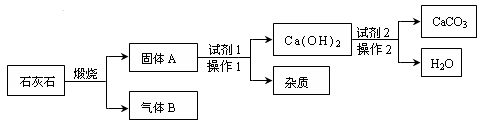
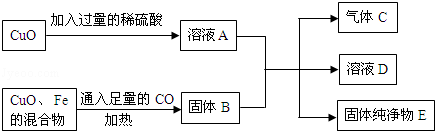
��1������ͼ�н��еIJ���1������2�������� ��
��2��д��ʯ��ʯ���յĻ�ѧ����ʽ ���÷�Ӧ�Ļ�����Ӧ������ ��
��3��������ͼ��ʾ�����У�����ˮ�ų������ȵ��������� ����������ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
��4��ʹԭ���и�ԭ�ӵ������ʾ����ܴﵽ100%�ǡ���ɫ��ѧ���ĺ�������֮һ�������������е����ʿ��������ã��������п�ѭ�����õ������� �����ѧʽ������

��1������ͼ�н��еIJ���1������2�������� ��
��2��д��ʯ��ʯ���յĻ�ѧ����ʽ ���÷�Ӧ�Ļ�����Ӧ������ ��
��3��������ͼ��ʾ�����У�����ˮ�ų������ȵ��������� ����������ˮ��Ӧ�Ļ�ѧ����ʽΪ ��
��4��ʹԭ���и�ԭ�ӵ������ʾ����ܴﵽ100%�ǡ���ɫ��ѧ���ĺ�������֮һ�������������е����ʿ��������ã��������п�ѭ�����õ������� �����ѧʽ������
��1������
��2��CaCO3 CaO + CO2�����д����÷֣� �ֽ⣨��Ӧ��
CaO + CO2�����д����÷֣� �ֽ⣨��Ӧ��
��3��CaO�������ƣ� CaO + H2O��Ca(OH)2
��4��CO2��H2O���жԵ�1�֣��д���1�֣�
��2��CaCO3
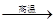 CaO + CO2�����д����÷֣� �ֽ⣨��Ӧ��
CaO + CO2�����д����÷֣� �ֽ⣨��Ӧ����3��CaO�������ƣ� CaO + H2O��Ca(OH)2
��4��CO2��H2O���жԵ�1�֣��д���1�֣�
���������ʯ��ʯ�к��в��������ʣ���ȥ���������ʷ����ǹ��ˣ�
ʯ��ʯ������������CaO �� CO2���÷�Ӧ���ڷֽⷴӦ��CaO �� CO2���������ﶼ����ˮ��Ӧ������CaO����ˮ�ų��������ȡ�ͨ������ͼ��֪��CO2��H2O��ѭ�����á�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ