��Ŀ����
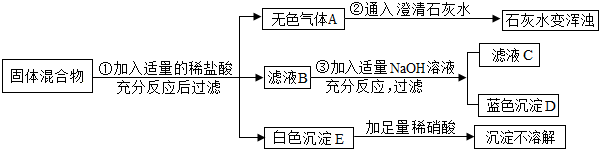
��6�֣���ij���������֪���п��ܺ���Na2CO3 ��BaCl2��CuO ��Na2SO4�� CaCO3���������е����ֻ���֡�����ͼ��ʾ����ʵ�飬���ֵ�������ͼ������������������з����ķ�Ӧ��ǡ����ȫ��Ӧ��
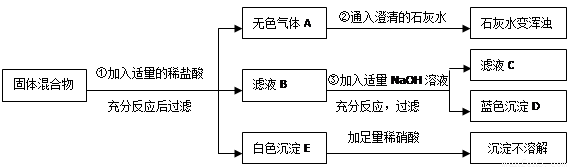
�Ը���ʵ����̺ͷ�����������д���¿հף�
����ɫ����D�Ļ�ѧʽΪ ��
��д��ʵ����̢��з�����Ӧ�Ļ�ѧ����ʽ ��
�ǹ��������У��������������У��϶����ڵ������� ������д����д�����÷֣���
������ҺC�У��϶����ڵ��������ǣ�д���ӷ��ţ� ��
�ɹ��������У��������������У�������ȷ���������� �� �ô˽��۵����� ��
��1��Cu(OH)2 ��2��CO2 +Ca(OH)2=CaCO3��+ H2O��3��BaCl2��Na2SO4��CuO
��4��Cl- ��5��CaCO3��Na2CO3 ��ֻдһ��Ҳ�÷֣� ��ΪCaCO3��Na2CO3 ������ϡ��
����������1���ɳ���ѧ����֪ʶ��֪����ɫ������������ͭ�����ԭ�������һ��������ͭ���ʴ�Ϊ��Cu��OH��2
��2��������̼���������Ʒ�Ӧ����̼��ư�ɫ������ˮ��ʯ��ˮ����ǣ��ʴ�Ϊ��CO2+Ca��OH��2=CaCO3��+H2O
��3����ɫ������������ͭ�����ԭ�������һ��������ͭ��������̼���������Ʒ�Ӧ����̼��ư�ɫ������ˮ��ʯ��ˮ����ǣ����ԭ�������Na2CO3��CaCO3������һ�֣���Ϊ��ɫ����E������ϡ���ᣬ���E�����ᱵ����ôԭ�������һ����BaCl2��Na2SO4���ʴ�Ϊ��BaCl2��CuO��Na2SO4����Na2CO3��CaCO3������һ�֣�
��4����Ϊ���������ᣬ�������ҺC�У��϶����ڵ��������������ӣ��ʴ�Ϊ��Cl-
��5��������ȷ����������Na2CO3��CaCO3�������������е�һ�֣��������߶��У���Ϊ���������ᷴӦ���ж�����̼����ų����ʴ�Ϊ��Na2CO3��CaCO3�����������ᷴӦ���ж�����̼����ų�

 ��ѧȫ��������ѵ��ϵ�д�
��ѧȫ��������ѵ��ϵ�д�