��Ŀ����
��2013?������һģ����ij���������֪�û�����п��ܺ���FeCl3��NaCl��NH4NO3��CuSO4���������е����ֻ���֣�Ϊ̽������ɣ���������ʵ�飨����������з����ķ�Ӧ��ǡ����ȫ��Ӧ����
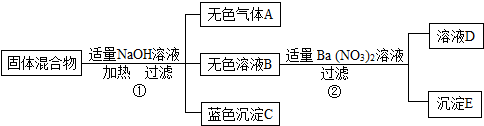
�Ը���ʵ����̺ͷ�����������д���¿հף�
��1����ʪ��ĺ�ɫʯ����ֽ��������A����ֽ��
��2������������������������У��϶������ڵ�������
��3������ҺD�У�һ�����е��������ǣ�д���ӷ��ţ�
��4��������з�����Ӧ�Ļ�ѧ����ʽΪ
��5������������������������У�������ȷ�����ڵ������ǣ�д��ѧʽ��

�Ը���ʵ����̺ͷ�����������д���¿հף�
��1����ʪ��ĺ�ɫʯ����ֽ��������A����ֽ��
��
��
ɫ��C�Ļ�ѧʽΪ��Cu��OH��2
Cu��OH��2
����2������������������������У��϶������ڵ�������
FeCl3
FeCl3
��д��ѧʽ������3������ҺD�У�һ�����е��������ǣ�д���ӷ��ţ�
NO3-
NO3-
����4��������з�����Ӧ�Ļ�ѧ����ʽΪ
Na2SO4+Ba��NO3��2�TBaSO4��+2NaNO3
Na2SO4+Ba��NO3��2�TBaSO4��+2NaNO3
����5������������������������У�������ȷ�����ڵ������ǣ�д��ѧʽ��
NaCl
NaCl
��Ҫ��һ��ȷ���ù�������ɷ֣�������ҺD��ʵ�飬���Ҫ˵��ʵ��������衢����������������ҺD�м�����������Һ�����ְ׳������μ�ϡ����������ܽ⣬˵��ԭ������к���NaCl���������ְ�ɫ��������ԭ������в�����NaCl
����ҺD�м�����������Һ�����ְ׳������μ�ϡ����������ܽ⣬˵��ԭ������к���NaCl���������ְ�ɫ��������ԭ������в�����NaCl
�����������ݰ�����ʹ��ɫʯ����Һ������������ͭ����ɫ�����������Ӻ���������ӡ�̼������ӻ����ɳ���������������Һ���Ի�ɫ���з���
����⣺��1���̬�����мӼ�����ɰ�������������ˮ�Լ��ԣ���ʹ��ɫ��ʯ�����ɫ��������ͭ����ɫ����������C�Ļ�ѧʽ�ǣ�Cu��OH��2��
��2�����������������Ȼ�����Ӧ���������������ɫ�������Ȼ��ƣ���ͼʾ��֪û�к��ɫ�������ɣ�˵���������Ȼ�����
��3����ɫ��ҺB�м������������ᱵ������������뱵�����������ᱵ����������һ�����ڵ�����������������ӣ�
��4��������з�����Ӧ�ķ�Ӧ���������ƺ����ᱵ�������������ᱵ�������ƣ��ù۲취��ƽ�����ᱵ������ϳ������ţ����Է���ʽ�ǣ�Na2SO4+Ba��NO3��2�TBaSO4��+2NaNO3��
��5�����������������������ɣ�˵��ԭ������к���笠����ӣ�����������泥�����ɫ��������˵������ͭ���ӣ�����������ͭ��û�к��ɫ�������ɣ�˵���������Ȼ��������Բ���ȷ�������Ȼ��ƣ����������ӵķ����ǣ�����ҺD�м�����������Һ�����ְ׳������μ�ϡ����������ܽ⣬˵��ԭ������к���NaCl���������ְ�ɫ��������ԭ������в�����NaCl��
�ʴ�Ϊ����1������ Cu��OH��2����2��FeCl3����3��NO3-����4��Na2SO4+Ba��NO3��2�TBaSO4��+2NaNO3����5��NaCl�� ����ҺD�м�����������Һ�����ְ׳������μ�ϡ����������ܽ⣬˵��ԭ������к���NaCl���������ְ�ɫ��������ԭ������в�����NaCl��
��2�����������������Ȼ�����Ӧ���������������ɫ�������Ȼ��ƣ���ͼʾ��֪û�к��ɫ�������ɣ�˵���������Ȼ�����
��3����ɫ��ҺB�м������������ᱵ������������뱵�����������ᱵ����������һ�����ڵ�����������������ӣ�
��4��������з�����Ӧ�ķ�Ӧ���������ƺ����ᱵ�������������ᱵ�������ƣ��ù۲취��ƽ�����ᱵ������ϳ������ţ����Է���ʽ�ǣ�Na2SO4+Ba��NO3��2�TBaSO4��+2NaNO3��
��5�����������������������ɣ�˵��ԭ������к���笠����ӣ�����������泥�����ɫ��������˵������ͭ���ӣ�����������ͭ��û�к��ɫ�������ɣ�˵���������Ȼ��������Բ���ȷ�������Ȼ��ƣ����������ӵķ����ǣ�����ҺD�м�����������Һ�����ְ׳������μ�ϡ����������ܽ⣬˵��ԭ������к���NaCl���������ְ�ɫ��������ԭ������в�����NaCl��
�ʴ�Ϊ����1������ Cu��OH��2����2��FeCl3����3��NO3-����4��Na2SO4+Ba��NO3��2�TBaSO4��+2NaNO3����5��NaCl�� ����ҺD�м�����������Һ�����ְ׳������μ�ϡ����������ܽ⣬˵��ԭ������к���NaCl���������ְ�ɫ��������ԭ������в�����NaCl��
�������ڽ������ʱ�����ȸ������������ȷ�����ڵ����ʣ�Ȼ���Ƶ���������е�������ж�����֤��

��ϰ��ϵ�д�
�����Ŀ