��Ŀ����
 ����ѧ��ѧ֪ʶ����������⣺
����ѧ��ѧ֪ʶ����������⣺��1�������ǵĺ�ˮ����ͼ��ʾ�ļ���ˮ�����о��������л���̿��������
����
����
��ͨ����װ�ô�������ˮ��
�����
�����
������������������2���;��ϵ������ü���ϴ�ྫ��ˮ����ϴ����ԭ����
ϴ�ྫ�����黯����
ϴ�ྫ�����黯����
����3���������������Ԫ����50���֣����к�������Ԫ����
��
��
����������ȱ��ijЩԪ��ʱ��Ӱ�콡�������¼���������ȱп��ص���C
C
��A��ƶѪ B�����Ͳ�C��٪��֢ D����״���״�
��������1�����ݻ���̿���������ԣ���������ζ��ɫ�صȽ��з������
��2��ϴ�ྫ��ϴ�Ӽ��������黯���ã�
��3�����������ڵ�Ԫ����ɡ�пԪ�ص��������ܺ�ȱ��֢���з������
��2��ϴ�ྫ��ϴ�Ӽ��������黯���ã�
��3�����������ڵ�Ԫ����ɡ�пԪ�ص��������ܺ�ȱ��֢���з������
����⣺��1������̿���������ԣ���������ζ��ɫ�أ������ǵĺ�ˮ�ü���ˮ�����о��������л���̿��������������ͨ����װ�ô�������ˮ�����п����Եĸ�þ������ȣ����ڻ���
��2��ϴ�ྫ��ϴ�Ӽ��������黯���ã��ܽ�����͵η�ɢ��ϸС���͵���ˮ���ߣ��ʲ;��ϵ������ü���ϴ�ྫ��ˮ����ϴ����
��3�������ں�������Ԫ������Ԫ�أ�пӰ�����巢����ȱп������ʳ�����������ٻ�������������٪��֢�ȣ�
�ʴ�Ϊ����1��������������2��ϴ�ྫ�����黯���ã���3������O��C��
��2��ϴ�ྫ��ϴ�Ӽ��������黯���ã��ܽ�����͵η�ɢ��ϸС���͵���ˮ���ߣ��ʲ;��ϵ������ü���ϴ�ྫ��ˮ����ϴ����
��3�������ں�������Ԫ������Ԫ�أ�пӰ�����巢����ȱп������ʳ�����������ٻ�������������٪��֢�ȣ�
�ʴ�Ϊ����1��������������2��ϴ�ྫ�����黯���ã���3������O��C��
��������������������ص�֪ʶ���п�������ȵ�֮һ�������ѶȲ����漰֪ʶ��϶࣬���������ѧ֪ʶ����ȷ�����Ĺؼ���

��ϰ��ϵ�д�
�����Ŀ
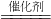 CH3OH����X�Ļ�ѧʽΪ________��
CH3OH����X�Ļ�ѧʽΪ________�� ��6�����پơ��к���һ�����ļ״�[CH3OH]�����ú��ʹ������Ѹ���½���ʧ����������������ҵ����ȡ�״��ķ���Ϊ��X + 2H2 ���� CH3OH����X�Ļ�ѧʽΪ ��
��6�����پơ��к���һ�����ļ״�[CH3OH]�����ú��ʹ������Ѹ���½���ʧ����������������ҵ����ȡ�״��ķ���Ϊ��X + 2H2 ���� CH3OH����X�Ļ�ѧʽΪ ��  CH3OH����X�Ļ�ѧʽΪ ��
CH3OH����X�Ļ�ѧʽΪ ��