��Ŀ����
����Ŀ����ˮ�廯�ƿ�������ȼ���������������������ˮ�������������ʡ�ʵ�����ù�ҵ����ʯ����������Al3+��Fe3+�����ʣ��Ʊ��廯�Ƶ���Ҫ�������£����������գ�
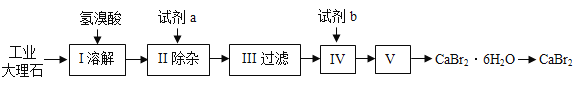
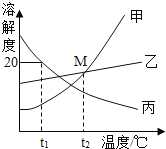
��1������ʹ�õ������ᣨHBr������������Ϊ26%������47%������������26%�������ᣬ����IJ��������в��������ձ���__________��
��2����֪��������Һ�в���NH4+������������Լ�a��_____��������Һ��pHԼΪ8.0����Al3+��Fe3+�ֱ�ת���ɳ�������ȥ��д�������Ļ�ѧʽ__________��
��3���Լ�b������__________��д�������Ļ�ѧ��Ӧ����ʽ____________________��
��4������������IJ���������_______________�����½ᾧ�����ˡ�
���𰸡���Ͳ�ͽ�ͷ�ι� Ca(OH)2 Al(OH)3��Fe(OH)3 ��ȥ������Ca(OH)2 Ca(OH)2 + 2HBr = CaBr2 + 2H2O ����Ũ��
��������
��1������47%������������26%�������ᣬ����Ũ��Һ��ϡ�����⣬ϡ����Һ����IJ��������в��������ձ�����Ͳ�ͽ�ͷ�ιܡ���Ҫ����ü�ˮ�������Ũ��Һ���������ȡһ������Һ�������Ҫ�õ����������У���Ͳ�ͽ�ͷ�ιܡ������Ͳ�ͽ�ͷ�ιܡ�
��2��������м�����������ǹ����ģ����Բ�����е���Һ�����Եģ�������Լ�a������Һ��pHԼΪ8.0���ܽ�Al3+��Fe3+�ֱ�ת���ɳ�������ȥ������a�Ǽͬʱ�������������ʣ�����a���������ƣ�ʯ��ˮ������Al3+��Fe3+�ֱ�ת�������������������������������Գ����Ļ�ѧʽΪAl(OH)3��Fe(OH)3�����Ca(OH)2��Al(OH)3��Fe(OH)3��
��3��������е���Һ�����Եģ�������Լ�a���������ƣ����������Բ�������Һ�к����������ƣ������Լ�bĿ�ij�ȥ�������ƻ��������������ʣ���b�������������ᡣ���������������Ʒ�Ӧ�����廯�ƺ�ˮ����Ӧ����ʽΪ��Ca(OH)2 + 2HBr = CaBr2 + 2H2O�������ȥ������Ca(OH)2��Ca(OH)2 + 2HBr = CaBr2 + 2H2O��
��4����������������������õ����Dz����������ʵ��廯����Һ���������������Ũ�������½ᾧ�����ˣ����Եõ��廯�ƾ��塣�������Ũ����

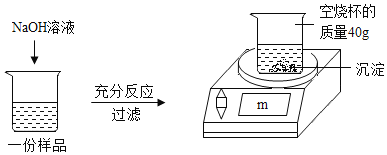
����Ŀ��Ϊ�ⶨijCuSO4��Һ�����ʵ�����������ȡ150g CuSO4��Һ��ƽ����Ϊ���ݣ�ÿ����Ʒ������ͼ��ʾ����ʵ�飬ʵ�����ݼ��±�������㣺
ʵ��1 | ʵ��2 | ʵ��3 | |
��Ʒ����/g | 50 | 50 | 50 |
NaOH��Һ����/g | 20 | 40 | 60 |
m/g | 42.45 | 44.9 | 44.9 |
��1��50g CuSO4��Һ��ȫ��Ӧʱ�����ó���������Ϊ_____g��
��2��CuSO4��Һ�����ʵ���������Ϊ_____��
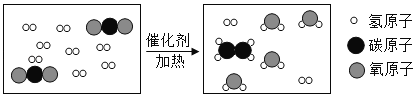
����Ŀ����ͼ��ijʳƷ��װ�еĸ������

��������ȤС��Ը������̽�����̣�����뵽����ȥ���ش������й����⣺
��1�����������ˮ��ԭ����_____���û�ѧ����ʽ��ʾ����
��2��ͬѧ�ǶԷ���һ��ʱ�������ijɷֽ���̽��
��������⣩��������ڵijɷ���ʲô��
�����룩����1������CaO ����2������Ca��OH��2 ����3������CaCO3
������ʵ�飩
ʵ�鲽�� | ʵ������ | ���� |
1��ȡ������������Թ��У����Թ��м���������ˮ�����ִ����Թ���� | �Թ�������ȸ� | ����_____ |
2������1��ˮ����Թ��е�����ɫ��̪��Һ | ��ɫ��̪��Һ���ɫ | ����Ca��OH��2 |
3������2��Ӧ����Թ��м���������______ | _____ | ����CaCO3����Ӧ�Ļ�ѧ����ʽ��______ |
��ʵ�������С��ͬѧ��Ϊ����2����ȷ��Ca��OH��2�Ƿ���ڣ�ԭ����_____��
���Ľ�ʵ�飩С��ͬѧ��������ʵ��

����С����ʵ������������к��е�������_____��