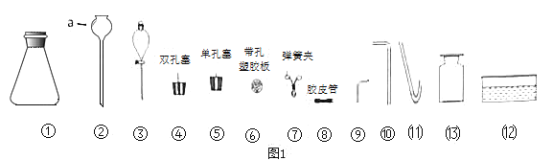
��Ŀ����
����Ŀ���������̲��˾�������Դ����Դ��
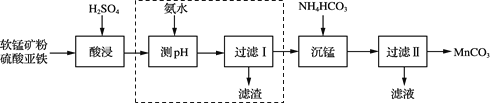
��1���ҹ��Ϻ���������ŷḻ�Ŀ�ȼ������ȼ������������Ҫ���м���ˮ����ɼ�����Ӻ�ˮ������ɣ�����������������̼�����ʡ�
�ټ��������ȼ�ϣ�����ȼ�յĻ�ѧ����ʽΪ________��
�ڻ�ѧ���ü����Ϊԭ���Ƴ��˽��ʯ��Ĥ���ñ仯����____������������������ѧ�����仯��
�۳��³�ѹʱ���ɼ����������������ɵĻ�����У�̼Ԫ�ص���������Ϊ75%�������������������____��
A H2��CO2 B H2��C3H8 C C3H8��C3H6 D CO��C3H8
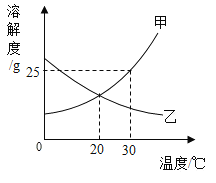
��2���о��Ӻ����еõ�������a��b��c���ܽ��ԡ�40��ʱ��ȡ������a��b��c�������ʵı�����Һ�������ձ���(״̬1)�����¶Ƚ���30��(״̬2)���������������ͼ1��ʾ��ͼ2Ϊa��b��c�������ʵ��ܽ�����ߡ�

��ϸ��ͼ1��ͼ2���ش��������⡣
��10��ʱ��a��b��c�������ʵ��ܽ���ɴ�С��˳����________��
���ձ�������____���ʵ���Һ��ѡ��a��b��c����
��30��ʱ���ձ�____����Һ��ˮ���������٣�ѡ��ס��ҡ�������
������˵����ȷ����____��
A ״̬1�������ձ����ܼ�������С��ϵΪ�ң��ף���
B ״̬1�������ձ�����������������С��ϵΪ�����ף���
C ״̬2�������ձ�����Һ������С��ϵΪ�ң��ף���
D ״̬2�������ձ�����������������С��ϵΪ�ף�������
���𰸡�CH4+2O2 ![]() 2H2O+CO2 ��ѧ BD c��b��a b �� ABC
2H2O+CO2 ��ѧ BD c��b��a b �� ABC
��������
��1���������ڿ����г��ȼ�����ɶ�����̼��ˮ����ѧ����ʽΪCH4+2O2 ![]() 2H2O+CO2��
2H2O+CO2��
���ü����Ϊԭ���Ƴ��˽��ʯ��Ĥ�������������ʣ��ñ仯���ڻ�ѧ�仯��
��������̼Ԫ�ص������ٷֱ�=![]() =75%��
=75%��
CO��̼Ԫ�ص������ٷֱ�=![]() ��42.9%��
��42.9%��
������̼��̼Ԫ�ص������ٷֱ�=![]() ��27.3%��
��27.3%��
C3H8��̼Ԫ�ص������ٷֱ�=![]() ��81.8%��
��81.8%��
C3H6��̼Ԫ�ص������ٷֱ�=![]() ��85.7%��
��85.7%��
��Ϊ��������̼Ԫ����������Ϊ75%��������=75%�����ԣ�����������ĺ�̼��һ��Ҫ����75% ��һ��ҪС��75%������ʹ���������̼Ԫ�����������ﵽ75%��B��H2��C3H8��D��CO��C2H4�����ԴﵽҪ��
��10��ʱ��a��b��c�������ʵ��ܽ���ɴ�С��˳����c��b��a��
��ab���ʵ��ܽ�����¶ȵ����߶���С�������¶�ab������Һ�����о�����������a���ܽ�����¶�Ӱ��ϴ�a��Һ�����ľ�����࣬���ձ�������b���ʵ���Һ���ձ�������a���ʵ���Һ��
���b��
��40��ʱ��a���ܽ�������ͬ������abc������Һ�У�a��Һ��ˮ��������С���¶Ƚ�����30��ʱ��ˮ���������䣬�ձ�������Һ��ˮ���������١�
���ձ�������b���ʵ���Һ���ձ�������c���ʵ���Һ���ձ�������a���ʵ���Һ��״̬1ʱ��a���ܽ�����c���ܽ����С��
A״̬1ʱ�������ձ����ܼ�������С��ϵΪ�ң��ף�������ȷ��
B ״̬1�������ձ�����������������С��ϵΪ�����ף��ң���ȷ��
C ״̬2ʱ���������Ĺ�����࣬��Һ������С����û�й�����������Һ���������ȷ��
D ״̬2�������ձ�����������������С��ϵΪ�����ף��ң��ʴ���
��ѡABC��

����Ŀ�����������ʵ����ܽ�����ش����⣺(��֪ˮ���ܶ�ԼΪ1g/cm3)
ʵ����� | ʵ���� |
ȡ100mlˮ������25g����أ����裬�ָ���20�� | ȫ���ܽ⣬����Һ�� |
�ټ�wg����أ����裬�ָ���20�� | ǡ�ñ��ͣ�����Һ�� |
�ټ�25g����أ����裬�ָ���20�� | ����Һ�� |
���� | ȫ���ܽ⣬����Һ�� |
��ȴ��20�� | �������壬����Һ�� |
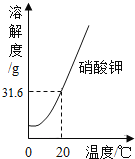
(1)w��ֵΪ___��
(2)һ�����ڲ�������Һ����___��(����ţ���ͬ)
(3)���ʵ���������һ����ȵ���___��