��Ŀ����
ˮ�DZ������Ȼ��Դ���ڹ�ũҵ�������ճ����������ż���㷺��Ӧ�á�
��1������ˮ���������̴�������ͼ��
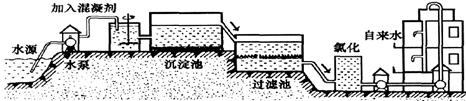
��ͼ���˳����л���̿������̿��_______���ã��ù��̷�����_______�仯���Ȼ�ʱ��ͨ��ͨ��һ��������������ˮ��Ӧ��������ʹ����ᣬ�䷴Ӧ��ѧ����ʽΪ___________________��ʵ��������AgNO3��Һʱ����������ˮ����ԭ���ǣ��û�ѧ����ʽ��ʾ��_______________��������أ�K2FeO4����һ�������ˮ�������������������Ԫ�صĻ��ϼ���________��
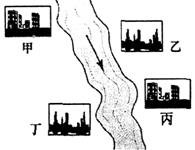
��2����ͼ���ں����мס��ҡ�����������������ÿ�������ų��ķ�Һֻ����Na2CO3��FeCl3��NaOH��HCl�е�һ�֡�ij����С��Ժ�ˮ���ʱ���֣��״���ˮ����ɫ���Ҵ���ˮ���к��ɫ�������ɣ�������ˮ�ɻ���壻�����������ݣ���ˮ���塣��ش�
�ټ����ų��ķ�Һ��һ�ּ������ʣ���������ʳʼ��Կ�ѡ�õ��Լ���_________��
���Ҵ���ˮ�к��ɫ�������ɵĻ�ѧ����ʽ��___________________________��
�۶����������ݵĻ�ѧ����ʽ��____________________________________��
��3������ˮ��Դ������Ӧ�ò�ȡ�Ĵ�ʩ��___________________________��
��1������ ���� AgNO3 + HCl = AgCl�� + HNO3��2������ɫ��̪����ɫʯ����Һ�� ��3NaOH + FeCl3 ====Fe(OH)3�� + 3NaCl����Na2CO3 + 2HCl === 2NaCl + CO2��+ H2O ��3����ũҵ�����м�����������ˮ����Ⱦ��IJ�����ũҵ������ʹ�ø�Ч�Ͷ���ũҩ�����ʡ�


 ��2012?��ɽ��һģ��ˮ�DZ������Ȼ��Դ���ڹ�ũҵ�������ճ��������й㷺��Ӧ�ã�
��2012?��ɽ��һģ��ˮ�DZ������Ȼ��Դ���ڹ�ũҵ�������ճ��������й㷺��Ӧ�ã�