��Ŀ����
ȼ������ᷢչ���������ŷdz���Ҫ�����ã�
��1�����������䡱�����ѻݼ�ǧ����
����Ȼ������Ҫ�ɷ��Ǽ��飬ʹ����Ȼ����ȼ���ܷ��������ЧӦ�ķ�����
��ͬ�¡�ͬѹ�£���ͬ����IJ�ͬ���������ͬ�ķ�������ԭ����ú��Ϊȼ�ϵļ�ͥ��Ҫ������Ȼ����ȼ�ϣ���ߵĸĽ�����Ϊ��
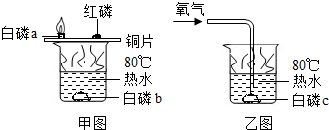
A�����ٿ����Ľ�������������Ȼ���Ľ����� B���������ߵĽ�����
C����������������������Ȼ���Ľ����� D���������ߵĽ�����
��2��ȼ��й©�����Σ�գ�ȼ����ȫ�Ǽ�ͥ�����ͷ�ȴ��£�Ϊ�˷�ֹȼ����ɢ������ȼ���м�����������������ζ������C2H5SH���������ȼ��ʱ����������̼�����������ˮ������д�����ȼ�յĻ�ѧ��Ӧ����ʽ��
��1�����������䡱�����ѻݼ�ǧ����
����Ȼ������Ҫ�ɷ��Ǽ��飬ʹ����Ȼ����ȼ���ܷ��������ЧӦ�ķ�����
����
����
����ܡ����ܡ����������ǣ�����ȼ���ж�����̼����
����ȼ���ж�����̼����
����ͬ�¡�ͬѹ�£���ͬ����IJ�ͬ���������ͬ�ķ�������ԭ����ú��Ϊȼ�ϵļ�ͥ��Ҫ������Ȼ����ȼ�ϣ���ߵĸĽ�����Ϊ��
C
C
A�����ٿ����Ľ�������������Ȼ���Ľ����� B���������ߵĽ�����
C����������������������Ȼ���Ľ����� D���������ߵĽ�����
��2��ȼ��й©�����Σ�գ�ȼ����ȫ�Ǽ�ͥ�����ͷ�ȴ��£�Ϊ�˷�ֹȼ����ɢ������ȼ���м�����������������ζ������C2H5SH���������ȼ��ʱ����������̼�����������ˮ������д�����ȼ�յĻ�ѧ��Ӧ����ʽ��
2C2H5SH+9O2
4CO2+6H2O+2SO2
| ||
2C2H5SH+9O2
4CO2+6H2O+2SO2
��
| ||
��������1���ٸ�����Ȼ��ȼ�յ���������������ЧӦ����Ҫ����������ڸ��ݷ�Ӧ��������P�������غ㶨�ɿ�����д��ѧ����ʽ�����������ṩ����Ϣ�ͻ�ѧ����ʽ���Խ�����ط�����жϣ�
��2�����������ȼ��ʱ����������̼�����������ˮ���н��
��2�����������ȼ��ʱ����������̼�����������ˮ���н��
����⣺��1����ʹ����Ȼ����ȼ�ϲ��ܴӸ����ϱ�������ЧӦ�ķ�������������Ȼ��ȼ�����ɶ�����̼���壬������̼���������ЧӦ����Ҫ���壻
���ɻ�ѧ����ʽ��֪��ÿ2���ܵ�ú����������1���������ӣ�ÿ2�������������4���������ӣ�ȼ����ͬ����Ĺܵ�ú������Ȼ�������Ŀ�������ϴ������Ȼ������ȼ�չܵ�ú������������ȼ��Ȼ������ߵĸĽ���������������������������Ȼ���Ľ���������ѡC��
��2�������ȼ��ʱ����������̼�����������ˮ����ѧ��Ӧ����ʽ2C2H5SH+9O2
4CO2+6H2O+2SO2��
�ʴ�Ϊ����1���ٲ��ܣ�����ȼ���ж�����̼���ɣ���C����2��2C2H5SH+9O2
4CO2+6H2O+2SO2��
���ɻ�ѧ����ʽ��֪��ÿ2���ܵ�ú����������1���������ӣ�ÿ2�������������4���������ӣ�ȼ����ͬ����Ĺܵ�ú������Ȼ�������Ŀ�������ϴ������Ȼ������ȼ�չܵ�ú������������ȼ��Ȼ������ߵĸĽ���������������������������Ȼ���Ľ���������ѡC��
��2�������ȼ��ʱ����������̼�����������ˮ����ѧ��Ӧ����ʽ2C2H5SH+9O2
| ||
�ʴ�Ϊ����1���ٲ��ܣ�����ȼ���ж�����̼���ɣ���C����2��2C2H5SH+9O2
| ||
������������Ҫ�����˻�ѧ����ʽ����д��ȼ��ȼ�շ���ıȽϵȷ����֪ʶ��

��ϰ��ϵ�д�
�����Ŀ
ȼ������ᷢչ������������Ҫ�����ã�
��1������ȼ�ϵı仯���������仯���� ��
A���ƾ��Ļӷ� B��ú��ȼ�� C�� ʯ������Һ��
��2���ƾ���Ϊȼ�ϵ�һ��������������Ҳ���Ź㷺Ӧ�ã��磺ͨ���ƾ��������ʾ����ɫ�仯�������ɿ��ٲ��˾���Ƿ�ƺ�ݳ�����Ӧԭ����2CrO3����ɫ��+3C2H5OH+3H2SO4�TCr2��SO4��3����ɫ��+3CH3CHO+6X��X�Ļ�ѧʽΪ ��
��3����������Ϊ��������ȼ�ϡ������û�ѧ����ʽ���͡�������������ԭ�� ��
��4����ͥȼ����ˮ���ϳ���ע��ȼ����ˮ������װ���ܱ�ԡ�һ�ͨ�粻�����������ͱ�ע�˱�ʶ��ԭ�� ��
��5��������Ȼ���֡�ֽ���������ͼ������ֽ���������ֽ�������������������ʢ�����ϣ�
���ƾ�ȼ��ʱֽ���ᱻ��ȼ�����ڴ��������н��Ͳ��������� ������ţ���
A�����������㣬ֽ����ȼ�� B��ֽ��ˮ��ʪ��ֽ���Ż�㽵��
C��ˮ����ʱ�����������¶ȴﲻ��ֽ���Ż�� D���ƾ�ȼ��ʱ���������٣��¶ȴﲻ��ֽ���Ż��
��6���±��г�����ÿ������ȼ����ȫȼ��ʱ�ų�����������Ԫ�ص�����������
�����������ݷ��������ѡ�õ�����ȼ���� ��ѡ������ԭ���� �� ��
��1������ȼ�ϵı仯���������仯����
A���ƾ��Ļӷ� B��ú��ȼ�� C�� ʯ������Һ��
��2���ƾ���Ϊȼ�ϵ�һ��������������Ҳ���Ź㷺Ӧ�ã��磺ͨ���ƾ��������ʾ����ɫ�仯�������ɿ��ٲ��˾���Ƿ�ƺ�ݳ�����Ӧԭ����2CrO3����ɫ��+3C2H5OH+3H2SO4�TCr2��SO4��3����ɫ��+3CH3CHO+6X��X�Ļ�ѧʽΪ
��3����������Ϊ��������ȼ�ϡ������û�ѧ����ʽ���͡�������������ԭ��
��4����ͥȼ����ˮ���ϳ���ע��ȼ����ˮ������װ���ܱ�ԡ�һ�ͨ�粻�����������ͱ�ע�˱�ʶ��ԭ��
��5��������Ȼ���֡�ֽ���������ͼ������ֽ���������ֽ�������������������ʢ�����ϣ�

���ƾ�ȼ��ʱֽ���ᱻ��ȼ�����ڴ��������н��Ͳ���������
A�����������㣬ֽ����ȼ�� B��ֽ��ˮ��ʪ��ֽ���Ż�㽵��
C��ˮ����ʱ�����������¶ȴﲻ��ֽ���Ż�� D���ƾ�ȼ��ʱ���������٣��¶ȴﲻ��ֽ���Ż��
��6���±��г�����ÿ������ȼ����ȫȼ��ʱ�ų�����������Ԫ�ص�����������
| ȼ������ | ���� | ���� | ���� | ��ϩ | ��Ȳ |
| ��ѧʽ | CH4 | C2H6 | C3H8 | C2H4 | C2H2 |
| ��������104J/g�� | 5.56 | 5.20 | 5.05 | 5.04 | 5.00 |
| ��Ԫ�ص�����������%�� | 25.0 | 20.0 | 18.2 | 14.3 | 7.7 |
��6�֣�ȼ������ᷢչ���������ŷdz���Ҫ�����á�
��1�����������䡱�����ѻݼ�ǧ����
����Ȼ������Ҫ�ɷ��Ǽ��飬ʹ����Ȼ����ȼ���ܷ��������ЧӦ�ķ���? (��ܡ����ܡ�)�������ǣ� ��
�� ͬ�¡�ͬѹ�£���ͬ����IJ�ͬ���������ͬ�ķ�������ԭ����ú��Ϊȼ�ϵļ�ͥ��Ҫ������Ȼ����ȼ�ϣ���ߵĸĽ�����Ϊ:
| A�����ٿ����Ľ�������������Ȼ���Ľ����� | B���������ߵĽ����� |
| C����������������������Ȼ���Ľ����� | D���������ߵĽ����� |